
Contributions
Abstract: PB1969
Type: Publication Only
Background
During many years, the combination of lenalidomide and dexamethasone (Rd) has been an effective treatment for patients with relapsed or refractory Multiple Myeloma (RRMM). On the basis of the available evidence, treatment with Rd may continue in responding patients until progression or unacceptable toxic effects. The data suggest full dose lenalidomide is important for optimal efficacy and to improve the progression free survival (PFS). Approaches to achieve higher doses of lenalidomide could include continuing therapy in responding patients and proactive adverse effects (AEs) management.
Aims
The main aim was to evaluate the incidence of two of most common non-hematologic AEs related to lenalidomide (rash and dystonia) in patients who received treatment with Rd. The second end points were to evaluate the response of rash after switching the enoxaparin to bemiparin and to evaluate the response of the dystonia after treatment with clonazepam, instead of lenalidomide dose reduction.
Methods
We retrospectively reviewed a consecutive cohort of patients with RRMM receiving Rd (R: 25 mg on days 1 through 21. d: 40 mg on days 1, 8, 15, and 22) in 28-day cycles until progression or unacceptable adverse effects, from 2011-2016. All patients received thromboprophylaxis with low-molecular-weight-heparin (LMWH) (Enoxaparin 40 mg subcutaneous daily) the first 4 cycles; thereafter, patients were switched to aspirin 100 mg in a day prophylaxis. Bemiparin 7500 anti-Xa IU once-daily dose was employed if enoxaparin was suspended. Clonazepam dose to treat dystonia was 0,5 mg twice daily. Data were analyzed with SPSS statistical v 22.0.
Results
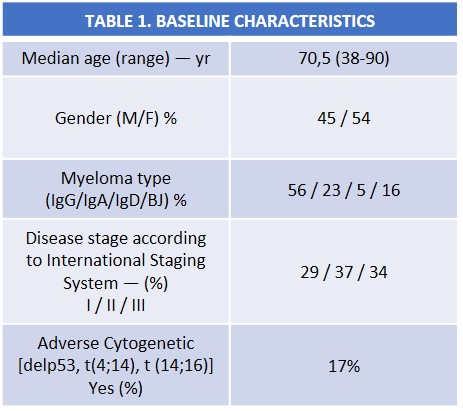
Between 2011 and 2016 a total of 65 patients received Rd in our center. Baseline characteristics are shown in Table 1. Patients received a median of 2 previous regimens (range 1-6). 51,5% of the patients had undergone one previous autologous stem-cell transplant (ASCT). Rash occurring in 12,3% of patients (grade 2), all of them were concurrently reciving enoxaparine. All rashes resolved switching the enoxaparine to bemiparine, maintaining same dose of lenalidomide. Neither treatment with esteroids or antihistaminic were administrated. Dystonias were reported in 23,1% of patients (grade 2), all of them dissapeared after treatment with clonazepam without lenalidomide dose reduction.
Conclusion
Rash and dystonias are frequent adverse effects of immunomodulatory drugs (IMiDs), particularly lenalidomide, often leading to treatment discontinuation and decreasing the potential benefits to patients.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Myeloma, Immunomodulatory thalidomide analog, Adverse reaction
Abstract: PB1969
Type: Publication Only
Background
During many years, the combination of lenalidomide and dexamethasone (Rd) has been an effective treatment for patients with relapsed or refractory Multiple Myeloma (RRMM). On the basis of the available evidence, treatment with Rd may continue in responding patients until progression or unacceptable toxic effects. The data suggest full dose lenalidomide is important for optimal efficacy and to improve the progression free survival (PFS). Approaches to achieve higher doses of lenalidomide could include continuing therapy in responding patients and proactive adverse effects (AEs) management.
Aims
The main aim was to evaluate the incidence of two of most common non-hematologic AEs related to lenalidomide (rash and dystonia) in patients who received treatment with Rd. The second end points were to evaluate the response of rash after switching the enoxaparin to bemiparin and to evaluate the response of the dystonia after treatment with clonazepam, instead of lenalidomide dose reduction.
Methods
We retrospectively reviewed a consecutive cohort of patients with RRMM receiving Rd (R: 25 mg on days 1 through 21. d: 40 mg on days 1, 8, 15, and 22) in 28-day cycles until progression or unacceptable adverse effects, from 2011-2016. All patients received thromboprophylaxis with low-molecular-weight-heparin (LMWH) (Enoxaparin 40 mg subcutaneous daily) the first 4 cycles; thereafter, patients were switched to aspirin 100 mg in a day prophylaxis. Bemiparin 7500 anti-Xa IU once-daily dose was employed if enoxaparin was suspended. Clonazepam dose to treat dystonia was 0,5 mg twice daily. Data were analyzed with SPSS statistical v 22.0.
Results
Between 2011 and 2016 a total of 65 patients received Rd in our center. Baseline characteristics are shown in Table 1. Patients received a median of 2 previous regimens (range 1-6). 51,5% of the patients had undergone one previous autologous stem-cell transplant (ASCT). Rash occurring in 12,3% of patients (grade 2), all of them were concurrently reciving enoxaparine. All rashes resolved switching the enoxaparine to bemiparine, maintaining same dose of lenalidomide. Neither treatment with esteroids or antihistaminic were administrated. Dystonias were reported in 23,1% of patients (grade 2), all of them dissapeared after treatment with clonazepam without lenalidomide dose reduction.

Conclusion
Rash and dystonias are frequent adverse effects of immunomodulatory drugs (IMiDs), particularly lenalidomide, often leading to treatment discontinuation and decreasing the potential benefits to patients.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Myeloma, Immunomodulatory thalidomide analog, Adverse reaction


