
Contributions
Abstract: PB1900
Type: Publication Only
Background
Patients with neutropenia, including those with haematologic malignancies, are at high risk of invasive fungal infections (IFI). Pre-2014, there were no formal written guidelines but the guidance at Poole Hospital NHS Foundation Trust specified the use of posaconazole oral suspension for primary prophylaxis in all high-risk patients except those with acute lymphoblastic leukaemia (ALL). In 2014 formal guideline changes included the introduction of the tablet formulation of posaconazole, use of micafungin as first line empirical therapy and a focus towards improving diagnostics to guide management. MSD Ltd. has developed the Fungal Service Evaluation Tool (FSET), a secure database and analysis tool, to support UK clinicians managing patients at risk of breakthrough IFI (BIFI) to evaluate their antifungal management.
Aims
This service evaluation aimed to utilise the FSET to evaluate the impact of the antifungal management guidelines on healthcare resource utilisation associated with patients at risk of a BIFI.
Methods
An interim analysis of high-risk adult patients with prolonged neutropenia aged ≥18 years at initiation of antifungal prophylaxis/treatment was carried out. Retrospective data on patient characteristics, antifungal prophylaxis and treatment, IFI-related diagnostic tests, hospital attendance/admission during antifungal prophylaxis were collected for 12-month periods before and after 2014 (Cohort 1: 2013; Cohort 2: 2015). Anonymised data was entered into the FSET and this data was analysed using descriptive statistics.
Results
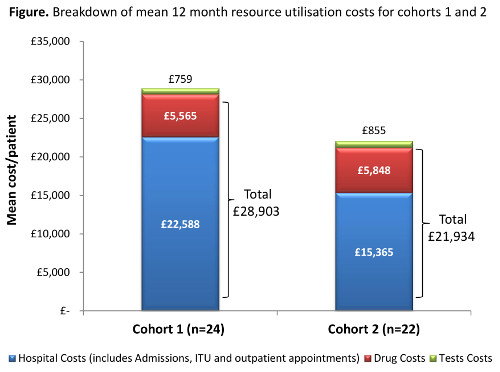
The evaluation included 24 patients in Cohort 1 (median age 66.8 [interquartile range (IQR): 47.5–72.2] years; 16 [67%] male; 5 [21%] ALL) and 22 patients in Cohort 2 (median age 66.8 [IQR: 51.7–73.4] years; 13 [59%] male; 1 [5%] ALL). At least one line of antifungal prophylaxis was recorded in 22 (92%) patients in Cohort 1 and 17 (71%) in Cohort 2. Posaconazole was the most commonly prescribed antifungal in Cohort 1 (18/24 [75%]) and Cohort 2 (17/22 [71%]). Other agents used included liposomal amphotericin B, fluconazole, and itraconazole. There were no patients in Cohort 1 and 2 (9%) patients in Cohort 2 (overall 4%) who experienced a BIFI: 1 was defined as confirmed and 1 as suspected. The mean 12 month costs per patient for all resource utilisation (including antifungal drug costs, hospitalisation costs [including admissions and attendances], investigations and tests) was £28,903 in Cohort 1 and £21,934 in Cohort 2 (figure 1). Hospitalisation costs were a key determinant of overall costs, which is common in the management of people with complex underlying disease. There were 4 (17%) patients in Cohort 1 and 1 (5%) in Cohort 2 who had a period of ITU associated stay, which typically has greater costs than general wards. The most common investigations/tests were blood cultures (Cohort 1: mean 13.8; Cohort 2: mean 10.7) and chest x-ray (Cohort 1: mean 4.0; Cohort 2: mean 2.5), which are in-line with routine clinical practice. Once implemented, the guideline was adhered to in the management of 19 patients (86%) in Cohort 2.
Conclusion
These data show that rates of breakthrough IFI are low in complex patients receiving antifungal prophylaxis/treatment. Furthermore, the results in Cohort 2 indicate that the switch to recommending posaconazole tablets did not result in an increase in the mean cost per patient of antifungal prophylaxis and shows a lower overall mean cost per patient. A larger cohort study over a longer period is warranted to confirm these findings.
Session topic: 29. Infectious diseases, supportive care
Keyword(s): neutropenia, Fungal infection, Cost analysis, Anti-fungal prophylaxis
Abstract: PB1900
Type: Publication Only
Background
Patients with neutropenia, including those with haematologic malignancies, are at high risk of invasive fungal infections (IFI). Pre-2014, there were no formal written guidelines but the guidance at Poole Hospital NHS Foundation Trust specified the use of posaconazole oral suspension for primary prophylaxis in all high-risk patients except those with acute lymphoblastic leukaemia (ALL). In 2014 formal guideline changes included the introduction of the tablet formulation of posaconazole, use of micafungin as first line empirical therapy and a focus towards improving diagnostics to guide management. MSD Ltd. has developed the Fungal Service Evaluation Tool (FSET), a secure database and analysis tool, to support UK clinicians managing patients at risk of breakthrough IFI (BIFI) to evaluate their antifungal management.
Aims
This service evaluation aimed to utilise the FSET to evaluate the impact of the antifungal management guidelines on healthcare resource utilisation associated with patients at risk of a BIFI.
Methods
An interim analysis of high-risk adult patients with prolonged neutropenia aged ≥18 years at initiation of antifungal prophylaxis/treatment was carried out. Retrospective data on patient characteristics, antifungal prophylaxis and treatment, IFI-related diagnostic tests, hospital attendance/admission during antifungal prophylaxis were collected for 12-month periods before and after 2014 (Cohort 1: 2013; Cohort 2: 2015). Anonymised data was entered into the FSET and this data was analysed using descriptive statistics.
Results
The evaluation included 24 patients in Cohort 1 (median age 66.8 [interquartile range (IQR): 47.5–72.2] years; 16 [67%] male; 5 [21%] ALL) and 22 patients in Cohort 2 (median age 66.8 [IQR: 51.7–73.4] years; 13 [59%] male; 1 [5%] ALL). At least one line of antifungal prophylaxis was recorded in 22 (92%) patients in Cohort 1 and 17 (71%) in Cohort 2. Posaconazole was the most commonly prescribed antifungal in Cohort 1 (18/24 [75%]) and Cohort 2 (17/22 [71%]). Other agents used included liposomal amphotericin B, fluconazole, and itraconazole. There were no patients in Cohort 1 and 2 (9%) patients in Cohort 2 (overall 4%) who experienced a BIFI: 1 was defined as confirmed and 1 as suspected. The mean 12 month costs per patient for all resource utilisation (including antifungal drug costs, hospitalisation costs [including admissions and attendances], investigations and tests) was £28,903 in Cohort 1 and £21,934 in Cohort 2 (figure 1). Hospitalisation costs were a key determinant of overall costs, which is common in the management of people with complex underlying disease. There were 4 (17%) patients in Cohort 1 and 1 (5%) in Cohort 2 who had a period of ITU associated stay, which typically has greater costs than general wards. The most common investigations/tests were blood cultures (Cohort 1: mean 13.8; Cohort 2: mean 10.7) and chest x-ray (Cohort 1: mean 4.0; Cohort 2: mean 2.5), which are in-line with routine clinical practice. Once implemented, the guideline was adhered to in the management of 19 patients (86%) in Cohort 2.

Conclusion
These data show that rates of breakthrough IFI are low in complex patients receiving antifungal prophylaxis/treatment. Furthermore, the results in Cohort 2 indicate that the switch to recommending posaconazole tablets did not result in an increase in the mean cost per patient of antifungal prophylaxis and shows a lower overall mean cost per patient. A larger cohort study over a longer period is warranted to confirm these findings.
Session topic: 29. Infectious diseases, supportive care
Keyword(s): neutropenia, Fungal infection, Cost analysis, Anti-fungal prophylaxis


