Abstract: PB1896
Type: Publication Only
Background
Hyperreactive malarial splenomegaly (HMS) is a common cause of massive splenomegaly in malarial-endemic areas. At present, diagnosis of patients with suspected HMS in tropical medicine departments of european hospitals is relatively frequent due to immigration and the return of missionaries and NGO workers after long periods in tropical countries.
Diagnostic protocols for HMS usually include a cytological study of bone marrow, because clinical similarities between HMS and lymphoproliferative disorders have been reported. However, there are no large series in the literature that estimate a bone marrow cytological standard associated to HMS. Another important issue is that patients with HMS are often multiinfected by other parasites, viruses and bacteria that may also induce splenomegaly.
Aims
The aim is to define the bone marrow cytological pattern of patients with confirmed HMS, as well as of HMS patients with associated viral (HIV, HBV, HCV) or parasitic diseases.
Methods
A retrospective cytological study of bone marrow aspirates from 95 patients with HMS (n=27), HMS+HIV (n=8), HMS+HCV/HBV (n=11) and HMS+intestinal parasitosis (n=49) has been performed.
Results
Bone marrow cellularity was normal in all the groups studied except in HMS+HIV patients, in which the cellularity was very diminished (statistically significant difference, p<0.01).
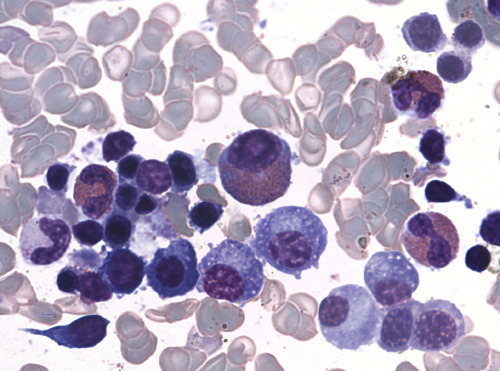
Most frequent alterations observed in all samples (HMS and HMS+other entities) that could define the HMS-bone marrow cytological pattern, were:
- Erythroid hyperplasia with dyserythropoiesis, which is reflected in a decreased myeloerythroid ratio.
- Increased eosinophils percentage.
- Increased lymphocytes percentage.
- Increased plasma cells percentage and detection of Mott cells in a significant proportion of samples from all series (48.1% of HMS samples).
Quantitative results for these variables are summarized in table 1.
Lymphocytosis was significantly increased in HMS+HBV/HCV bone marrow (p=0.04). Significant detection of atypical lymphocytes (>4%) varied widely between the groups, ranging from 14.8% of HMS bone marrows to 75.0% of HMS+HIV bone marrows (statistically significant difference, p<0.01). There was no lymphoma evidence in any case.
No quantitative or qualitative alterations were detected in megakaryocytes, except for a slight decrease in HMS+HIV bone marrows (statistically non-significant difference).
| Reference values | HMS | HMS+HIV | HMS+HBV/HCV | HMS+IP |
Myelo:erytroid ratio | 3.5:1 | 2.0:1 ± 0.8 | 2.2:1 ± 1.3 | 2.4:1 ± 0.6 | 2.5:1 ± 0.7 |
Eosinophils (%) | < 5 | 12.4 ± 10.0 | 8.6 ± 4.9 | 9.4 ± 8.9 | 12.3 ± 8.3 |
Lymphocytes (%) | 10 – 16 | 23.0 ± 9.5 | 26.7 ± 9.2 | 27.6 ± 5.7 | 23.8 ± 7.0 |
Plasma cells (%) | ≤ 4 | 6.8 ± 2.7 | 8.1 ± 3.1 | 6.4 ± 1.6 | 7.3 ± 3.0 |
Table 1. Quantitative results (mean ± standard deviation)
Conclusion
As far as we know, this is the largest series of HMS bone marrow analyzed. Identification of common cytological findings in all the groups studied allows defining a characteristic cytological pattern for HMS. The reason for these findings could be related to an aberrant chronic immune response caused by a continuous exposure to malaria parasites. Only bone marrows of HIV coinfected patients present additional specific alterations (decreased cellularity and high proportion of atypical lymphocytes).
Some authors hypothesize that HMS could eventually evolve to chronic lymphocytic leukemia, hairy cell leukemia or splenic lymphoma with villous lymphocytes, so a special follow-up would be advisable for those patients with a high proportion of atypical lymphocytes.
Session topic: 29. Infectious diseases, supportive care





