Abstract: PB1861
Type: Publication Only
Background
The prognosis of Hodgkin lymphoma (HL) has improved significantly with the implementation of a risk-adapted treatment that combines chemo and radiotherapy. Altough this approach has led to the greatest advance in disease response, the benefit in terms of overall survival (OS) has been jeopardized by long term toxicity.
The identification of risk factors is crucial to assign each patient to a well defined risk group and prevent under or overtreatment, minimizing the risk of relapse and long term toxicity.
Aims
To analyze the risk factors associated with survival in HL treated with an ABVD based regimen that restricted radiotherapy only to bulky disease.
Methods
We retrospectively analyzed HL patients diagnosed in 4 centers in Tarragona area (Catalonia, Spain), between 1995 and 2015, treated uniformly according to a local protocol.
Patients were assigned into 4 groups: G1: favorable early stage: ABVDx6 cycles, G2: Bulky early stage without other risk factors: ABVDx6 + IFRDT. G3: unfavorable early stage (B symptoms) and advanced stage without bulky disease: ABVDx8, G4: Bulky advanced stage: AVBDx8 + IFRDT
Results
A total of 183 patients were analyzed with a median follow up of 82 months [range 1-244]. Male/female ratio was 1,29. Median age was 36 years [range 16-82]. Complete response was achieved in 160 patients (87,4%). The estimated OS at 20 years for the whole group was 62.7%.
Kaplan–Meier method and log rank test were used for survival analysis. Cox proportional hazard model was used for univariate analysis to identify predictive factors for OS. Factors with significance (p <0.05) were considered for multivariate Cox regression.
In univariate analysis, worse OS was found in patients with increased LDH, non-NS subtype, albumin <3,5 g/dL, B symptoms, HIV+, advance stage and ESR >50 mm (log rank p=0,012; p=0,049; p=0,024; p=0,002; p=0,005; p=0,004 and p=0,001 respectively).
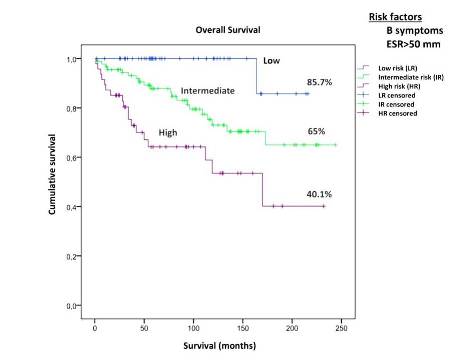
The multivariate Cox regression analysis identified B symptoms and ESR >50 mm as independent prognostic factors for OS (p=0,002; p=0,006 respectively).
These variables allowed us to identify 3 patient groups: low (no risk factors), intermediate (either B symtoms or ESR>50 mm) and high risk (both risk factors), with significant differences in OS. Estimation for OS was uniformly analyzed at 216 months (18 years), which is the shortest follow up period for patients in the low risk group.
Patients in the low, intermediate and high risk groups had an estimated OS of 85.7%, 65% and 40.1% (p<0.001)
Conclusion
B symptoms and ESR>50mm are independently associated with OS. The combination of these factors can stratify patients in low, intermediate and high risk groups with significant differences in OS, regardless their clinical stage.
Session topic: 17. Hodgkin lymphoma - Clinical





