
Contributions
Abstract: PB1838
Type: Publication Only
Background
The tyrosine kinase inhibitor (TKI) imatinib was the first targeted therapy for patients with chronic-phase chronic myeloid leukemia (CP-CML), and its introduction was associated with substantial improvements in response and survival compared to previous therapies. Earlier studies have indicated that the effect of age at diagnosis of CP-CML was minimized in patients treated with imatinib: fewer responses but the same outcome for older patients. However, recently published results from clinical controlled trials indicated that there were differences in clinical outcome depending on age at diagnosis of CP-CML.
Aims
The aim of this study was to evaluate impact of age on the treatment outcome in patients with chronic myeloid leukemia treated with frontline imatinib.
Methods
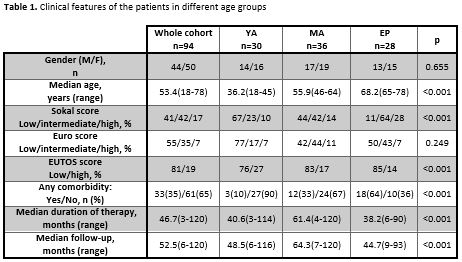
A newly diagnosed CP-CML patients treated and followed in our institution were surveyed retrospectively from August 2006 to August 2016. According to age, cohort was divided into three groups: young adults (18-45 years) (YA), middle aged adults (46-64 years) (MA) and elderly persons (65 and more years) (EP). Patients’ demographics, disease risk scores, duration of imatinib therapy and follow-up, cytogenetic and molecular responses, adverse event (AEs), the 5-year event-free survival (EFS) and 5-year overall survival (OS) were all evaluated. Clinical features of the patients in different age groups are summarized in Table 1.
Results
The patient cohort consisted of 94 patients with median age of 53.4 years (range 18-78), with a slight predominance of females of 53.2%. There were more patients with intermediate and high Sokal scores in the EP group than in the groups MA and YA (p<0.001). To the contrary of that, most patients with high EUTOS score were observed in the group YA compared to MA and EP groups (p<0.001). The three groups were balanced regarding Euro score. The median duration of imatinib therapy was the longest in MA group (61.4 months vs. 40.6 months in YA and 38.2 months in EP patients p<0.001). Furthermore, median follow-up duration was also the longest in MA group (64.3 months vs. 48.5 months in YA and 44.7 months in EP patients p<0.001). The rates of complete cytogenetic response (CCyR) were similar in all three analysed groups (80.6% in YA, 86.5% in MA and 75.9% in EP, p=0.328) while rate of major molecular response was the highest in the MA group (83.3% vs. 63.3% in YA and 57.1% in EL, p=0.001). The percentages of patients who switched to second-generation TKIs were similar in all three groups (36.7% in YA vs. 30% in MA vs. 32.1% in EP, p=0.559). There were the most of non-hematological AEs all grades in EP group (25% vs. 13.3% in YA and 13.8% in MA, p=0.005). Hematological AEs also were common in EP group but not statistically significant (17.8% vs. 10% in YA and in 12.1% in MA, p=0.156). The 5-years EFS in the MA group (88% (95%CI 82.1-96.9)) was significantly higher than in YA group (65.3% (95%CI 59.1-78.1)) and in EP group (60.2% (95%CI 49.5-73.7)). The 5-years OS in the EP group (74.7% (95%CI 65.9-89.0)) was significantly lower than in YA group (93.1% (95%CI 87.2-99.5)) and in MA group (90.8% (95%CI 85.8-97.8)). The number of deaths, both CML related or not related, was the largest in the EP group (25% vs. 13.3% in YA and 13.8% in MA, p<0.001).
Conclusion
Results of this study indicate that age at diagnosis impacts the course of chronic myeloid leukemia treated with imatinib. The best clinical outcomes have middle age patients in terms of the highest rates achieved optimal therapeutic response and longer survival without events and overall survival. The degree of therapeutic responds in the elderly is comparable with that observed in younger patients, but the presence of comorbidity and more frequent occurrence of adverse events were affecting relatively lower overall survival. Although it might be expected that younger patient population has a better clinical outcome than patients middle age, a possible cause of poor outcomes is probably a late diagnosis at an advanced stage of the disease.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): imatinib, Chronic myeloid leukemia, Age, Outcome
Abstract: PB1838
Type: Publication Only
Background
The tyrosine kinase inhibitor (TKI) imatinib was the first targeted therapy for patients with chronic-phase chronic myeloid leukemia (CP-CML), and its introduction was associated with substantial improvements in response and survival compared to previous therapies. Earlier studies have indicated that the effect of age at diagnosis of CP-CML was minimized in patients treated with imatinib: fewer responses but the same outcome for older patients. However, recently published results from clinical controlled trials indicated that there were differences in clinical outcome depending on age at diagnosis of CP-CML.
Aims
The aim of this study was to evaluate impact of age on the treatment outcome in patients with chronic myeloid leukemia treated with frontline imatinib.
Methods
A newly diagnosed CP-CML patients treated and followed in our institution were surveyed retrospectively from August 2006 to August 2016. According to age, cohort was divided into three groups: young adults (18-45 years) (YA), middle aged adults (46-64 years) (MA) and elderly persons (65 and more years) (EP). Patients’ demographics, disease risk scores, duration of imatinib therapy and follow-up, cytogenetic and molecular responses, adverse event (AEs), the 5-year event-free survival (EFS) and 5-year overall survival (OS) were all evaluated. Clinical features of the patients in different age groups are summarized in Table 1.
Results
The patient cohort consisted of 94 patients with median age of 53.4 years (range 18-78), with a slight predominance of females of 53.2%. There were more patients with intermediate and high Sokal scores in the EP group than in the groups MA and YA (p<0.001). To the contrary of that, most patients with high EUTOS score were observed in the group YA compared to MA and EP groups (p<0.001). The three groups were balanced regarding Euro score. The median duration of imatinib therapy was the longest in MA group (61.4 months vs. 40.6 months in YA and 38.2 months in EP patients p<0.001). Furthermore, median follow-up duration was also the longest in MA group (64.3 months vs. 48.5 months in YA and 44.7 months in EP patients p<0.001). The rates of complete cytogenetic response (CCyR) were similar in all three analysed groups (80.6% in YA, 86.5% in MA and 75.9% in EP, p=0.328) while rate of major molecular response was the highest in the MA group (83.3% vs. 63.3% in YA and 57.1% in EL, p=0.001). The percentages of patients who switched to second-generation TKIs were similar in all three groups (36.7% in YA vs. 30% in MA vs. 32.1% in EP, p=0.559). There were the most of non-hematological AEs all grades in EP group (25% vs. 13.3% in YA and 13.8% in MA, p=0.005). Hematological AEs also were common in EP group but not statistically significant (17.8% vs. 10% in YA and in 12.1% in MA, p=0.156). The 5-years EFS in the MA group (88% (95%CI 82.1-96.9)) was significantly higher than in YA group (65.3% (95%CI 59.1-78.1)) and in EP group (60.2% (95%CI 49.5-73.7)). The 5-years OS in the EP group (74.7% (95%CI 65.9-89.0)) was significantly lower than in YA group (93.1% (95%CI 87.2-99.5)) and in MA group (90.8% (95%CI 85.8-97.8)). The number of deaths, both CML related or not related, was the largest in the EP group (25% vs. 13.3% in YA and 13.8% in MA, p<0.001).

Conclusion
Results of this study indicate that age at diagnosis impacts the course of chronic myeloid leukemia treated with imatinib. The best clinical outcomes have middle age patients in terms of the highest rates achieved optimal therapeutic response and longer survival without events and overall survival. The degree of therapeutic responds in the elderly is comparable with that observed in younger patients, but the presence of comorbidity and more frequent occurrence of adverse events were affecting relatively lower overall survival. Although it might be expected that younger patient population has a better clinical outcome than patients middle age, a possible cause of poor outcomes is probably a late diagnosis at an advanced stage of the disease.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): imatinib, Chronic myeloid leukemia, Age, Outcome


