
Contributions
Abstract: PB1834
Type: Publication Only
Background
Resistance to tyrosine kinase inhibitors (TKI) in patients with chronic myeloid leukemia (CML) is frequently caused by point mutations in the BCR-ABL kinase domain, including the gatekeeper mutant T315I, which confers a high degree of resistance to all currently approved tyrosine kinase inhibitors except ponatinib. The role of allo-HSCT in such patients is still disputable.
Aims
To evaluate the results of different treatment modalities in CML patients with T315I mutation
Methods
Retrospective analysis of 53 BCR-ABLT315I –positive CML patients (pts) was done.
Results
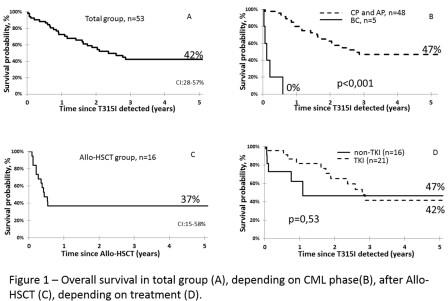
Me follow-up time after T315I detection was 21 months (1-100). 5-years OS in whole group was 42% (fig.1A). According to multivariate analysis only CML phase at the time of mutation detection significantly affect to survival in whole group. All pts in BC (n=5, 2 in HSCT group and 3 in non-HSCT group) died within first year after T315I indication wherein Me survival time was 1,3 month (fig.1B). 5-years OS in non-HSCT group (n=37) was 42% with Me survival time 2,8 years. 5-years OS after allo-HSCT (n=16) was 37% with Me survival time 5 months (fig.1C). All living patients after allo-HSCT are in deep molecular response. There was no significant difference in 5-years OS between TKI (n=21) and non-TKI (n=16) pharmacological therapy (non-HSCT) groups (42% and 47% respectively, p=0,53) (fig.1D).
Conclusion
Detection of T315I mutation in TKI-resistant patients is extremely unfavorable factor for survival, especially in the advanced phase CML, and it is a great reason for switching to ponatinib or other new potential investigated drugs if possible. Allo-HSCT can be a potential option for this group of patients in case of good selection taking into consideration transplant risk, especially for patients in CP ≥2.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): Resistance, Mutation, BCR-ABL, Allo BMT
Abstract: PB1834
Type: Publication Only
Background
Resistance to tyrosine kinase inhibitors (TKI) in patients with chronic myeloid leukemia (CML) is frequently caused by point mutations in the BCR-ABL kinase domain, including the gatekeeper mutant T315I, which confers a high degree of resistance to all currently approved tyrosine kinase inhibitors except ponatinib. The role of allo-HSCT in such patients is still disputable.
Aims
To evaluate the results of different treatment modalities in CML patients with T315I mutation
Methods
Retrospective analysis of 53 BCR-ABLT315I –positive CML patients (pts) was done.
Results
Me follow-up time after T315I detection was 21 months (1-100). 5-years OS in whole group was 42% (fig.1A). According to multivariate analysis only CML phase at the time of mutation detection significantly affect to survival in whole group. All pts in BC (n=5, 2 in HSCT group and 3 in non-HSCT group) died within first year after T315I indication wherein Me survival time was 1,3 month (fig.1B). 5-years OS in non-HSCT group (n=37) was 42% with Me survival time 2,8 years. 5-years OS after allo-HSCT (n=16) was 37% with Me survival time 5 months (fig.1C). All living patients after allo-HSCT are in deep molecular response. There was no significant difference in 5-years OS between TKI (n=21) and non-TKI (n=16) pharmacological therapy (non-HSCT) groups (42% and 47% respectively, p=0,53) (fig.1D).

Conclusion
Detection of T315I mutation in TKI-resistant patients is extremely unfavorable factor for survival, especially in the advanced phase CML, and it is a great reason for switching to ponatinib or other new potential investigated drugs if possible. Allo-HSCT can be a potential option for this group of patients in case of good selection taking into consideration transplant risk, especially for patients in CP ≥2.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): Resistance, Mutation, BCR-ABL, Allo BMT


