SHOULD SWITCHING TO SECOND GENERATION TKIS BE A RULE IN PATIENTS WITH CP-CML AFTER 3-6 MONTHS OF IMATINIB TREATMENT: RETROSPECTIVE ANALYSIS OF CML PATIENTS TREATED IN A SINGLE BRAZILIAN CANCER CENTER
(Abstract release date: 05/18/17)
EHA Library. Pifano Soares A. 05/18/17; 182544; PB1830

Amanda Pifano Soares
Contributions
Contributions
Abstract
Abstract: PB1830
Type: Publication Only
Background
Early molecular response is an important predictor for survival and therapy-free remission in chronic myeloid leukemia (CML). The current guidelines define BCR-ABL1 >10% at 3 months and/or 1-10% at 6 months as warning signs; however, it is not clear if switching imatinib to second generation TKIs in this scenario improves responses and overall survival in patients outside clinical trials.
Aims
To analyze the proportion of patients with major molecular response (MMR) at 12 months according to the molecular response at 3 and 6 months in a cohort of CML population, not enrolled in clinical trials and treated only with imatinib. Also evaluate the incidence of molecular responses log3.0, log4.0 and log4.5 at any time in patients who did not switch to second generation TKIs.
Methods
Retrospective analysis of all 226 patients diagnosed with CML from January 2007 until January 2015 in our hospital. The exclusions criterias were: advanced phases, inclusion in clinical trial, treatment with second-generation TKI in the first 12 months (due to toxicity or failure). The molecular response was evaluated according ELN recommendations: RQ-PCR assessment of BCR-ABL1 levels every 3 months until achievement of MMR, with molecular evaluation every 3-6 months afterward. All samples were analyzed in the same laboratory which was standardized since 2007.
Results
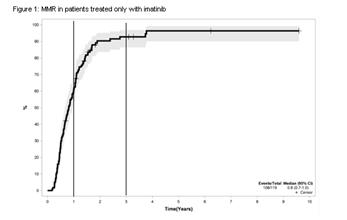
In the first cohort, 150 patients with CML chronic phase were analyzed. Optimal molecular responses by the ELN at 3 and 6 months were predictors of MMR by 12 months (94% vs. 6%, p<0.0001 at 3m, 89.3% vs. 10.7%, p<0.0001 at 6m), but there was no overall survival benefit. A second cohort with 119 patients received only imatinib, with a medium follow-up time of 71 months (13-117m). MMR was achieved by 60% of this imatinib-only group after 12 months and by more than 90% after 36 months (figure 1). Patients with BCR-ABL1 <10% at 3 months and/or <1% had a higher probability of achieving MMR3, MMR4 and MMR4.5 at any time.
Conclusion
Our study shows that around 30% of the patients that do not fail to imatinib at the first year of treatment may be late responders. Not all patients should change therapy, if they have not reached MMR at 12 months. Molecular response at 3 at 6 months might guide the decision to switch TKI, but patient’s comorbities, possibility of discontinuation and cost of therapy should also be considered.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): Molecular response, imatinib, Chronic myeloid leukemia
Abstract: PB1830
Type: Publication Only
Background
Early molecular response is an important predictor for survival and therapy-free remission in chronic myeloid leukemia (CML). The current guidelines define BCR-ABL1 >10% at 3 months and/or 1-10% at 6 months as warning signs; however, it is not clear if switching imatinib to second generation TKIs in this scenario improves responses and overall survival in patients outside clinical trials.
Aims
To analyze the proportion of patients with major molecular response (MMR) at 12 months according to the molecular response at 3 and 6 months in a cohort of CML population, not enrolled in clinical trials and treated only with imatinib. Also evaluate the incidence of molecular responses log3.0, log4.0 and log4.5 at any time in patients who did not switch to second generation TKIs.
Methods
Retrospective analysis of all 226 patients diagnosed with CML from January 2007 until January 2015 in our hospital. The exclusions criterias were: advanced phases, inclusion in clinical trial, treatment with second-generation TKI in the first 12 months (due to toxicity or failure). The molecular response was evaluated according ELN recommendations: RQ-PCR assessment of BCR-ABL1 levels every 3 months until achievement of MMR, with molecular evaluation every 3-6 months afterward. All samples were analyzed in the same laboratory which was standardized since 2007.
Results
In the first cohort, 150 patients with CML chronic phase were analyzed. Optimal molecular responses by the ELN at 3 and 6 months were predictors of MMR by 12 months (94% vs. 6%, p<0.0001 at 3m, 89.3% vs. 10.7%, p<0.0001 at 6m), but there was no overall survival benefit. A second cohort with 119 patients received only imatinib, with a medium follow-up time of 71 months (13-117m). MMR was achieved by 60% of this imatinib-only group after 12 months and by more than 90% after 36 months (figure 1). Patients with BCR-ABL1 <10% at 3 months and/or <1% had a higher probability of achieving MMR3, MMR4 and MMR4.5 at any time.

Conclusion
Our study shows that around 30% of the patients that do not fail to imatinib at the first year of treatment may be late responders. Not all patients should change therapy, if they have not reached MMR at 12 months. Molecular response at 3 at 6 months might guide the decision to switch TKI, but patient’s comorbities, possibility of discontinuation and cost of therapy should also be considered.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): Molecular response, imatinib, Chronic myeloid leukemia
{{ help_message }}
{{filter}}


