
Contributions
Abstract: PB1816
Type: Publication Only
Background
Pulmonary hypertension (PH) has been reported as a serious adverse event in chronic myeloid leukemia (CML) patients treated by dasatinib. French group reported incidence of PH diagnosed by cardiac catheterization as 0.45% (13 of 2,900 patients) in symptomatic patients treated with dasatinib. Dasatinib-related PH usually resolves after cessation of treatment, but it can be fatal, as two deaths in France and one in Japan have been documented.
Aims
To clarify the incidence of tyrosine kinase inhibitor (TKI)-related PH, we noninvasively screened CML patients who have been given imatinib, nilotinib or dasatinib by echocardiography.
Methods
105 patients with CML in chronic phase (CP) who received TKI were enrolled in this study between 2014 and 2015. Nine patients with newly diagnosed CML in CP prior to TKI treatment were added as control. Patients underwent echocardiography to evaluate values of tricuspid regurgitation pressure gradient (TRPG), which relates to severity of PH. Patients with TRPG values >31mmHg were suspected of PH onset according to European Society of Cardiology criteria. All patients gave informed consent.
Results
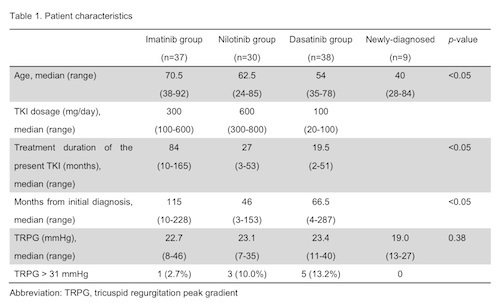
Patients were divided into 3 groups by the TKIs they used at the time of study enrollment; 37 patients on imatinib, 30 on nilotinib and 38 on dasatinib (Table). In imatinib group, patients’ age was significantly higher, and duration of treatment was also longer than those of the 2nd generation TKIs. Echocardiography revealed mean values of TRPG as 22.7, 23.1 and 23.4mmHg in imatinib, nilotinib and dasatinib groups, respectively (p=0.887), and these values were higher than that in the newly diagnosed CML patients (19.0mmHg), though without significance (p=0.38). Nine of the 105 patients (8.6%) presented with an elevated TRPG>31mmHg, suggesting the presence of PH; 1 of 37 (2.7%) in imatinib group, 3 of 30 (10.0%) in nilotinib group, and 5 of 38 (13.2%) in dasatinib group. Three patients complained of dyspnea, while the remaining 6 were asymptomatic. We found no apparent risk factors associated with TRPG elevation, however, there were trends toward correlation of age and TRPG values in nilotinib and dasatinib treated patients, and treatment duration and TRPG values in nilotinib treated patients. Imatinib dosage tended to inversely correlate with TRPG value, suggesting that imatinib might decrease pulmonary arterial blood pressure in a dose-dependent manner.
Conclusion
PH is a rare but life-threatening adverse event for dasatinib-treated patients, and its definitive diagnosis is made by cardiac catheterization. However, cardiac catheterization is too invasive for PH screening of the many patients with TKIs who do not have any symptoms. Our study, by using echocardiography, detected TRPG elevation not only in dasatinib treated patients (13.2%) but also in imatinib (2.7%) and nilotinib (10%), including patients without any symptoms. This indicates possible PH onset among patients treated with imatinib or nilotinib, as well as with dasatinib. Although TRGP values obtained by echocardiography might not be fully compatible with those by cardiac catheterization, the results suggested that noninvasive echocardiography is sensitive for screening PH and is also effective for easily screening groups of patients with suspect subclinical PH among patients treated with any available TKIs. Careful screening with echocardiography is necessary especially for older patients who have received TKIs for a long time.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): Pulmonary hypertension, Tyrosine kinase inhibitor
Abstract: PB1816
Type: Publication Only
Background
Pulmonary hypertension (PH) has been reported as a serious adverse event in chronic myeloid leukemia (CML) patients treated by dasatinib. French group reported incidence of PH diagnosed by cardiac catheterization as 0.45% (13 of 2,900 patients) in symptomatic patients treated with dasatinib. Dasatinib-related PH usually resolves after cessation of treatment, but it can be fatal, as two deaths in France and one in Japan have been documented.
Aims
To clarify the incidence of tyrosine kinase inhibitor (TKI)-related PH, we noninvasively screened CML patients who have been given imatinib, nilotinib or dasatinib by echocardiography.
Methods
105 patients with CML in chronic phase (CP) who received TKI were enrolled in this study between 2014 and 2015. Nine patients with newly diagnosed CML in CP prior to TKI treatment were added as control. Patients underwent echocardiography to evaluate values of tricuspid regurgitation pressure gradient (TRPG), which relates to severity of PH. Patients with TRPG values >31mmHg were suspected of PH onset according to European Society of Cardiology criteria. All patients gave informed consent.
Results
Patients were divided into 3 groups by the TKIs they used at the time of study enrollment; 37 patients on imatinib, 30 on nilotinib and 38 on dasatinib (Table). In imatinib group, patients’ age was significantly higher, and duration of treatment was also longer than those of the 2nd generation TKIs. Echocardiography revealed mean values of TRPG as 22.7, 23.1 and 23.4mmHg in imatinib, nilotinib and dasatinib groups, respectively (p=0.887), and these values were higher than that in the newly diagnosed CML patients (19.0mmHg), though without significance (p=0.38). Nine of the 105 patients (8.6%) presented with an elevated TRPG>31mmHg, suggesting the presence of PH; 1 of 37 (2.7%) in imatinib group, 3 of 30 (10.0%) in nilotinib group, and 5 of 38 (13.2%) in dasatinib group. Three patients complained of dyspnea, while the remaining 6 were asymptomatic. We found no apparent risk factors associated with TRPG elevation, however, there were trends toward correlation of age and TRPG values in nilotinib and dasatinib treated patients, and treatment duration and TRPG values in nilotinib treated patients. Imatinib dosage tended to inversely correlate with TRPG value, suggesting that imatinib might decrease pulmonary arterial blood pressure in a dose-dependent manner.

Conclusion
PH is a rare but life-threatening adverse event for dasatinib-treated patients, and its definitive diagnosis is made by cardiac catheterization. However, cardiac catheterization is too invasive for PH screening of the many patients with TKIs who do not have any symptoms. Our study, by using echocardiography, detected TRPG elevation not only in dasatinib treated patients (13.2%) but also in imatinib (2.7%) and nilotinib (10%), including patients without any symptoms. This indicates possible PH onset among patients treated with imatinib or nilotinib, as well as with dasatinib. Although TRGP values obtained by echocardiography might not be fully compatible with those by cardiac catheterization, the results suggested that noninvasive echocardiography is sensitive for screening PH and is also effective for easily screening groups of patients with suspect subclinical PH among patients treated with any available TKIs. Careful screening with echocardiography is necessary especially for older patients who have received TKIs for a long time.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): Pulmonary hypertension, Tyrosine kinase inhibitor


