E14A2 TRANSCRIPT IS ASSOCIATED WITH HIGHER PROBABILITY OF DURABLE TREATMENT FREE REMISSION IN CML PATIENTS
(Abstract release date: 05/18/17)
EHA Library. d adda M. 05/18/17; 182528; PB1814

Dr. Mariella d adda
Contributions
Contributions
Abstract
Abstract: PB1814
Type: Publication Only
Background
TKIs discontinuation in CML-CP patients with deep molecular response (DMR) are feasible, safe and 40-60% of them maintain treatment free remission (TFR); sokal risk score and duration of TKI-therapy were significantly associated with molecular relapse, according to Euro-Ski and STIM1 trials. While it is known that patients with e14a2 achieve earlier, deeper and more durable responses compared to those with e13a2, few information is available on the influence of the type of bcr-abl transcript on TFR duration
Aims
Here we describe our single center experience of TKI discontinuation in CML-CP patients with sustained DMR
Methods
Bcr-abl transcripts were determined by RQ-PCR analysis performed in accordance with EAC protocol (Gabert et al, Leukemia 2003) and to the standards of the Italian national network Labnet. All 174 CML-CP patients presently followed at our institution according to ELN guidelines and treated with 1st or 2nd TKIs were analysed: 103 (59%) had e14a2 and 69 (40%) e13a2 transcript (in 2 pz bcr-abl were not detectable). Criteria for TKI discontinuation was sustained DMR (MR4 or better) for at least 2 years. After TKI withdrawal, RQ-PCR for BCR-ABL was performed every month during the first year and every 2 months thereafter. TKI treatment was reintroduced immediately if DMR loss occurred. TFR was defined as the time between the date of TKI cessation and the date of restarting treatment for DMR loss or, if TKI was not resumed, the date of the last contact
Results
Forty-nine patients, 25 male and 24 female, discontinued TKI treatment. At the time of discontinuation median age was 63 years (43-85), median time from TKI start 113 months (30-172), median duration of sustained DMR 60 months (24-153). Sokal distribution was 49%, 29% and 20% for low, intermediate and high risk (one patient was not evaluable). Among our 174 patients 39% (40/103) of all e14a2 patients and 13% (9/69) of all e13a2 discontinued TKI (P 0.0002, chi square). Thirty-six patients discontinued imatinib (11 of them with previous INF treatment), 13 stopped nilotinib (8 in first line, 5 in second line treatment). Median follow up after treatment discontinuation was 19 months (3-76), including 31 patients with follow up > 12 months. Thirteen (26%) patients lost DMR. Median time off-therapy for these patients was 3 months (2-8), and only 1 lost DMR after 6 months. Therapy was restarted in all 13 patients (2 in MR1, 4 in MR2, 7 in MR3), 10 achieved a second DMR after a median interval of 2 months (1-7); 2/13 patients are in M3 after 7 and 12 months, 1 patient is not yet evaluable. Univariate analysis showed no difference in relapse risk according to age, gender, type and duration of TKI, duration of stable DMR and sokal score risk. Ten out of 11 patients treated with INF before imatinib remained in TFR. Of note, the type of bcr-abl transcript was significantly linked to DMR loss: after TKI discontinuation, 32/40 e14a2 patients (78%) maintained DMR vs 4/9 e13a2 patients (42%) (p 0.03). After 12 months 78% (+/-6% CI95%) of e14a2 and 41,6 (+/-17% CI95%) of e13a2 patients were still in TFR (log-rank: P=0.033) (see figure). Using multivariate analysis the type of bcr-abl transcript and previous INF treatment correlated with DMR loss (p 0.012 and p 0.033). One patient died during follow up in DMR for CML-unrelated cause
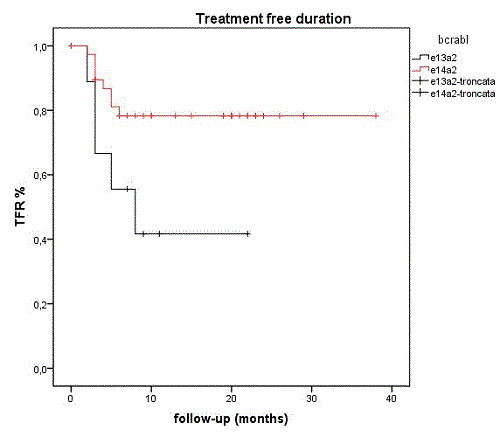
Conclusion
In e14a2 CML patients the probability of discontinuation for sustained DMR is significantly higher as compared with e13a2 patients. Moreover, after discontinuation, e14a2 have significantly lower probability of DMR loss than e13a2. These data confirm that e14a2 transcript is associated with a more favorable CML disease profile than e13a2 (Jain et al., Blood 2016); in addition they show that e14a2 is a favorable prognostic factor for TFR maintenance
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): Quantitative RT-PCR, Chronic myeloid leukemia, BCR-ABL
Abstract: PB1814
Type: Publication Only
Background
TKIs discontinuation in CML-CP patients with deep molecular response (DMR) are feasible, safe and 40-60% of them maintain treatment free remission (TFR); sokal risk score and duration of TKI-therapy were significantly associated with molecular relapse, according to Euro-Ski and STIM1 trials. While it is known that patients with e14a2 achieve earlier, deeper and more durable responses compared to those with e13a2, few information is available on the influence of the type of bcr-abl transcript on TFR duration
Aims
Here we describe our single center experience of TKI discontinuation in CML-CP patients with sustained DMR
Methods
Bcr-abl transcripts were determined by RQ-PCR analysis performed in accordance with EAC protocol (Gabert et al, Leukemia 2003) and to the standards of the Italian national network Labnet. All 174 CML-CP patients presently followed at our institution according to ELN guidelines and treated with 1st or 2nd TKIs were analysed: 103 (59%) had e14a2 and 69 (40%) e13a2 transcript (in 2 pz bcr-abl were not detectable). Criteria for TKI discontinuation was sustained DMR (MR4 or better) for at least 2 years. After TKI withdrawal, RQ-PCR for BCR-ABL was performed every month during the first year and every 2 months thereafter. TKI treatment was reintroduced immediately if DMR loss occurred. TFR was defined as the time between the date of TKI cessation and the date of restarting treatment for DMR loss or, if TKI was not resumed, the date of the last contact
Results
Forty-nine patients, 25 male and 24 female, discontinued TKI treatment. At the time of discontinuation median age was 63 years (43-85), median time from TKI start 113 months (30-172), median duration of sustained DMR 60 months (24-153). Sokal distribution was 49%, 29% and 20% for low, intermediate and high risk (one patient was not evaluable). Among our 174 patients 39% (40/103) of all e14a2 patients and 13% (9/69) of all e13a2 discontinued TKI (P 0.0002, chi square). Thirty-six patients discontinued imatinib (11 of them with previous INF treatment), 13 stopped nilotinib (8 in first line, 5 in second line treatment). Median follow up after treatment discontinuation was 19 months (3-76), including 31 patients with follow up > 12 months. Thirteen (26%) patients lost DMR. Median time off-therapy for these patients was 3 months (2-8), and only 1 lost DMR after 6 months. Therapy was restarted in all 13 patients (2 in MR1, 4 in MR2, 7 in MR3), 10 achieved a second DMR after a median interval of 2 months (1-7); 2/13 patients are in M3 after 7 and 12 months, 1 patient is not yet evaluable. Univariate analysis showed no difference in relapse risk according to age, gender, type and duration of TKI, duration of stable DMR and sokal score risk. Ten out of 11 patients treated with INF before imatinib remained in TFR. Of note, the type of bcr-abl transcript was significantly linked to DMR loss: after TKI discontinuation, 32/40 e14a2 patients (78%) maintained DMR vs 4/9 e13a2 patients (42%) (p 0.03). After 12 months 78% (+/-6% CI95%) of e14a2 and 41,6 (+/-17% CI95%) of e13a2 patients were still in TFR (log-rank: P=0.033) (see figure). Using multivariate analysis the type of bcr-abl transcript and previous INF treatment correlated with DMR loss (p 0.012 and p 0.033). One patient died during follow up in DMR for CML-unrelated cause

Conclusion
In e14a2 CML patients the probability of discontinuation for sustained DMR is significantly higher as compared with e13a2 patients. Moreover, after discontinuation, e14a2 have significantly lower probability of DMR loss than e13a2. These data confirm that e14a2 transcript is associated with a more favorable CML disease profile than e13a2 (Jain et al., Blood 2016); in addition they show that e14a2 is a favorable prognostic factor for TFR maintenance
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): Quantitative RT-PCR, Chronic myeloid leukemia, BCR-ABL
{{ help_message }}
{{filter}}


