Abstract: PB1782
Type: Publication Only
Background
Over the last decades, progress in chronic lymphocytic leukemia (CLL) treatment has resulted in an impressive increase in overall survival (OS). In CLL, as in other tumors, response to therapy overcomes negative prognostic factors and is the most important predictor of survival. Clinical stages reflect tumor load and correlate with OS both at diagnosis and over the course of the disease (Rai et al, Blood 1975).
Aims
To determine whether changes in clinical stage discriminate patients with different outcome within IWCLL response categories, particularly the heterogenous partial response (PR) group (Hallek et al, Blood 2008).
Methods
Two-hundred twenty-nine patients with CLL were retrospectively evaluated. Median follow-up was 91 months (range, 2-390). CLL diagnosis was based on IWCLL criteria. Endpoints were time to next treatment (TTT) and OS. TTT and OS curves were estimated by the Kaplan-Meier method and differences were evaluated with the log-rank test. Response to therapy was determined according to IWCLL recommendations and by changes in clinical stage A landmark analysis was performed in ninety-two patients in whom a PR was achieved at any time during the course of the disease, using the time when a PR was achieved as “time 0”.
Results
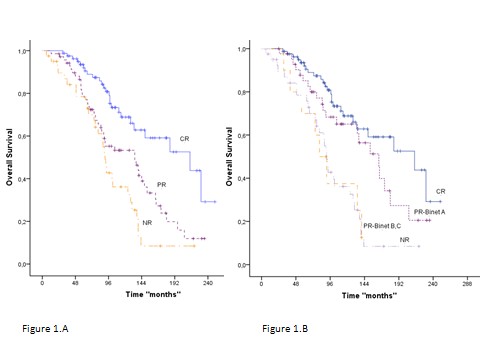
From the whole series of 229 patients, those who achieved a better IWCLL degree of response after first line of therapy had a better OS than those with an inferior response (p<0.001). With a median follow up of 91 months (range, 2-390), the median survival in patients who achieved complete remission (CR) was 214 (95% CI: 123-305) vs. 134 (95% CI: 79-189) and 91 (95% CI: 84-98) months in those who achieved PR and failed to therapy, respectively (Figure 1.A).
Among patients in PR (n=66), after a median follow-up of 42.5 months (range 1-201), those patients with stage A disease at the time of response evaluation (PR Binet A) had significantly better outcome than those whose stage was Binet B/C (median survival 63 vs. 43 months; p= 0.047).
Interestingly, when the analysis was restricted to response assessment after first line therapy (n=229), patients who achieved PR Binet A did not have significant differences in OS compared to those patients who were in CR (median survival were 164 and 214 months respectively; p<0.001); on the contrary, patients in PR Binet B/C had a similar outcome than those who did not respond to treatment (median survival 81 and 91 months respectively) (Figure 1.B). Similar results were observed in the outcome of patients with PR subclassified according to Rai clinical stage.
Conclusion
Changes in clinical stage provide reliable information on the degree of response to therapy in patients with CLL, particularly those in the IWCLL PR category. This study supports the use of clinical stages as a complementary and simple tool to assess response in patients with CLL, both at the end and over the course of treatment.
Session topic: 6. Chronic lymphocytic leukemia and related disorders - Clinical





