
Contributions
Abstract: PB1760
Type: Publication Only
Background
Patients affected by chronic lymphocytic leukemia (CLL) that have trisomy 12 (+12) on FISH analysis have unique clinical and biological features. In a prior analysis (Autore F, ASH 2016) of 487 patients with +12 compared to 816 patients with negative FISH, patients with +12 had a significantly higher prevalence of elevated LDH, β-2-microglobulin, ZAP70 positivity, CD38 positivity, CD49d positivity and unmutated IGHV as compared to patients with negative FISH. They also showed shorter progression free survival (PFS), treatment free survival (TFS) and overall survival (OS).
Aims
To identify clinical and laboratory features that predict disease progression, time to treatment and survival in treatment-naive patients with +12 CLL.
Methods
Results
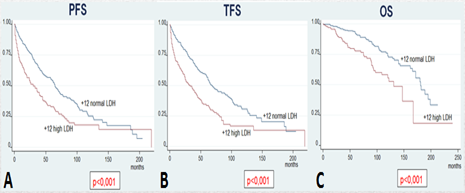
Conclusion
Our study on 487 patients with +12 CLL and the analysis on 250 patients of the validation cohort showed that patients with +12 and elevated LDH have shorter PFS, TFS, OS and CLL-specific survival.
Session topic: 5. Chronic lymphocytic leukemia and related disorders - Biology
Keyword(s): Prognostic factor, FISH, Chronic Lymphocytic Leukemia
Abstract: PB1760
Type: Publication Only
Background
Patients affected by chronic lymphocytic leukemia (CLL) that have trisomy 12 (+12) on FISH analysis have unique clinical and biological features. In a prior analysis (Autore F, ASH 2016) of 487 patients with +12 compared to 816 patients with negative FISH, patients with +12 had a significantly higher prevalence of elevated LDH, β-2-microglobulin, ZAP70 positivity, CD38 positivity, CD49d positivity and unmutated IGHV as compared to patients with negative FISH. They also showed shorter progression free survival (PFS), treatment free survival (TFS) and overall survival (OS).
Aims
To identify clinical and laboratory features that predict disease progression, time to treatment and survival in treatment-naive patients with +12 CLL.
Methods
Results

Conclusion
Our study on 487 patients with +12 CLL and the analysis on 250 patients of the validation cohort showed that patients with +12 and elevated LDH have shorter PFS, TFS, OS and CLL-specific survival.
Session topic: 5. Chronic lymphocytic leukemia and related disorders - Biology
Keyword(s): Prognostic factor, FISH, Chronic Lymphocytic Leukemia


