
Contributions
Abstract: PB1646
Type: Publication Only
Background
Despite of targeted effects of tyrosine kinase inhibitors (TKIs), they are not absolutely selective in relation to their target. Hair pigmentation is regulated by factors including the interaction of the ligand stem cell factor (SCF) with its class III receptor tyrosine kinase, c-kit. Hair depigmentation observes during therapy TKI with action directed against class III receptor tyrosine kinase (PDGFRα, PDGFRβ, C-KIT, CSF1R, FLT3). But other TKI such as BCR/Abl TKI can also inhibit class III receptor tyrosine kinase by non-targeted actions. Skin reactions are the most common observed during the epidermal growth factor receptor-tyrosine kinase inhibitor treatment.
Aims
To describe the spectrum of skin and hair reactions in patients with acute leukemias (Ph+/Ph- acute lymphoblastic leukemia and acute myeloid leukemia) during the treatment by second-generation TKI (bosutinib), third-generation TKI (ponatinib) and multikinase inhibitor (sorafenib).
Methods
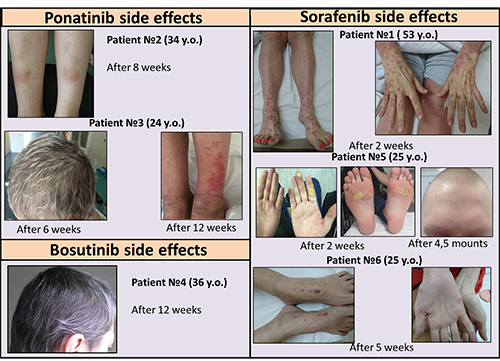
From 2016 to March 2017 6 patients (pts), age 24-53 (median 29,5), 1 male, 5 female, received second or third line therapy with target tyrosine kinase inhibitors in National Research center for Hematology. One pt (pt 1) with AML had been receiving chemotherapy (decitabine, cytarabine, idarubicin) with continuous treatment of sorafenib. Three pts with Ph+ ALL received TKIs. Two of them with T315I mutation (pts 2, 3) received ponatinib and one pt (pt 4), without molecular remission on dasatinib and nilotinib therapy, received second-generation TKI (bosutinib). One pt with B-ALL was treated by sorafenib due to refractory disease on the first-line therapy (pt 5). And one patient (pt 6) with T-cell ALL received sorafenib with nelarabine containing chemotherapy due to early relapse after allogeneic stem cell transplantation.
Results
All of the 6 patients who had taken second-generation TKI (bosutinib), third-generation TKI (ponatinib) and multikinase inhibitor sorafenib developed dermatologic reactions (skin rash or grey hair). Generalized maculo-papular skin rash grade II evolved after two weeks of sorafenib treatment in pt1. Both patients on ponatinib therapy developed localized maculo-papular skin rash grade I in pt 2 after 8 weeks of therapy. In pt 3 after 6 weeks of ponatinib treatment gray hair observed. Skin rash with pigmentation grade I evolved in pt 3 after 12 weeks of therapy. Pt 4 had gray hair after 12 weeks second-generation TKI (bosutinib) treatment. Palmar-plantar erythrodysesthesia syndrome grade II and hair and total skin depigmentation were evolved after 2 weeks and after 4,5 months respectively observed during the sorafenib treatment in pt 5 (with psoriasis anamnesis). Pt 6 developed localized maculo-papular skin rash grade I after 5 weeks of sorafenib treatment. Despite of all patients developed dermatological side effects, temporarily discontinuation of TKI therapy was required in only three (50%) cases. In the other cases the treatment was continued. The therapy was restarted in all pts with temporarily discontinuation after skin lesions disappearing.
Conclusion
Dermatological adverse events in acute leukemia pts who have taken second-generation TKI (bosutinib), third-generation TKI (ponatinib) and multikinase inhibitor sorafenib they were not serious. Temporarily dose reduction or interrupting of TKI therapy led to complete regression of skin lesions. Restarting TKI at full dose did not lead to dermatological adverse reactions reappearing. Moreover, the temporary cancellation did not reduce its effectiveness.
Session topic: 2. Acute lymphoblastic leukemia - Clinical
Keyword(s): Tyrosine kinase inhibitor, Adverse reaction, acute leukemia
Abstract: PB1646
Type: Publication Only
Background
Despite of targeted effects of tyrosine kinase inhibitors (TKIs), they are not absolutely selective in relation to their target. Hair pigmentation is regulated by factors including the interaction of the ligand stem cell factor (SCF) with its class III receptor tyrosine kinase, c-kit. Hair depigmentation observes during therapy TKI with action directed against class III receptor tyrosine kinase (PDGFRα, PDGFRβ, C-KIT, CSF1R, FLT3). But other TKI such as BCR/Abl TKI can also inhibit class III receptor tyrosine kinase by non-targeted actions. Skin reactions are the most common observed during the epidermal growth factor receptor-tyrosine kinase inhibitor treatment.
Aims
To describe the spectrum of skin and hair reactions in patients with acute leukemias (Ph+/Ph- acute lymphoblastic leukemia and acute myeloid leukemia) during the treatment by second-generation TKI (bosutinib), third-generation TKI (ponatinib) and multikinase inhibitor (sorafenib).
Methods
From 2016 to March 2017 6 patients (pts), age 24-53 (median 29,5), 1 male, 5 female, received second or third line therapy with target tyrosine kinase inhibitors in National Research center for Hematology. One pt (pt 1) with AML had been receiving chemotherapy (decitabine, cytarabine, idarubicin) with continuous treatment of sorafenib. Three pts with Ph+ ALL received TKIs. Two of them with T315I mutation (pts 2, 3) received ponatinib and one pt (pt 4), without molecular remission on dasatinib and nilotinib therapy, received second-generation TKI (bosutinib). One pt with B-ALL was treated by sorafenib due to refractory disease on the first-line therapy (pt 5). And one patient (pt 6) with T-cell ALL received sorafenib with nelarabine containing chemotherapy due to early relapse after allogeneic stem cell transplantation.
Results
All of the 6 patients who had taken second-generation TKI (bosutinib), third-generation TKI (ponatinib) and multikinase inhibitor sorafenib developed dermatologic reactions (skin rash or grey hair). Generalized maculo-papular skin rash grade II evolved after two weeks of sorafenib treatment in pt1. Both patients on ponatinib therapy developed localized maculo-papular skin rash grade I in pt 2 after 8 weeks of therapy. In pt 3 after 6 weeks of ponatinib treatment gray hair observed. Skin rash with pigmentation grade I evolved in pt 3 after 12 weeks of therapy. Pt 4 had gray hair after 12 weeks second-generation TKI (bosutinib) treatment. Palmar-plantar erythrodysesthesia syndrome grade II and hair and total skin depigmentation were evolved after 2 weeks and after 4,5 months respectively observed during the sorafenib treatment in pt 5 (with psoriasis anamnesis). Pt 6 developed localized maculo-papular skin rash grade I after 5 weeks of sorafenib treatment. Despite of all patients developed dermatological side effects, temporarily discontinuation of TKI therapy was required in only three (50%) cases. In the other cases the treatment was continued. The therapy was restarted in all pts with temporarily discontinuation after skin lesions disappearing.

Conclusion
Dermatological adverse events in acute leukemia pts who have taken second-generation TKI (bosutinib), third-generation TKI (ponatinib) and multikinase inhibitor sorafenib they were not serious. Temporarily dose reduction or interrupting of TKI therapy led to complete regression of skin lesions. Restarting TKI at full dose did not lead to dermatological adverse reactions reappearing. Moreover, the temporary cancellation did not reduce its effectiveness.
Session topic: 2. Acute lymphoblastic leukemia - Clinical
Keyword(s): Tyrosine kinase inhibitor, Adverse reaction, acute leukemia


