
Contributions
Abstract: PB1644
Type: Publication Only
Background
Aims
To characterize long-term survival outcomes including leukemia-free survival (LFS) and overall survival (OS) for Ph+ adult ALL patients treated with imatinib versus dasatinib.
Methods
Results
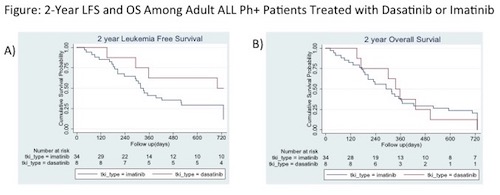
Among 46 patients with Ph+ ALL, 74% (n=34) were in imatinib group and 17% (n=8) in dasatinib group; 9% were treated with other or no TKI (1 ponatinib and 3 with no TKI). Thirty-eight percent (n=13) of patients in imatinib group and 13% (n=1) in dasatinib group switched to a different TKI due to adverse effects or failure to achieve remission. There was a trend towards increased 2-year LFS for patients on dasatinib (HR 0.40, 95% CI: 0.14-1.14, p=0.09) and no difference in 2-year mortality (HR 1.00 95%CI: 0.46-2.17, p=0.99). Molecular response data was available for 61% (n=28) of patients; 75% of imatinib group achieved CMR or MMR (65% CMR) compared to 76% of dasatinib group (63% CMR) (p=0.98).
Conclusion
Session topic: 2. Acute lymphoblastic leukemia - Clinical
Keyword(s): Survival, Philadelphia chromosome, Acute lymphoblastic leukemia, Tyrosine kinase inhibitor
Abstract: PB1644
Type: Publication Only
Background
Aims
To characterize long-term survival outcomes including leukemia-free survival (LFS) and overall survival (OS) for Ph+ adult ALL patients treated with imatinib versus dasatinib.
Methods
Results
Among 46 patients with Ph+ ALL, 74% (n=34) were in imatinib group and 17% (n=8) in dasatinib group; 9% were treated with other or no TKI (1 ponatinib and 3 with no TKI). Thirty-eight percent (n=13) of patients in imatinib group and 13% (n=1) in dasatinib group switched to a different TKI due to adverse effects or failure to achieve remission. There was a trend towards increased 2-year LFS for patients on dasatinib (HR 0.40, 95% CI: 0.14-1.14, p=0.09) and no difference in 2-year mortality (HR 1.00 95%CI: 0.46-2.17, p=0.99). Molecular response data was available for 61% (n=28) of patients; 75% of imatinib group achieved CMR or MMR (65% CMR) compared to 76% of dasatinib group (63% CMR) (p=0.98).

Conclusion
Session topic: 2. Acute lymphoblastic leukemia - Clinical
Keyword(s): Survival, Philadelphia chromosome, Acute lymphoblastic leukemia, Tyrosine kinase inhibitor


