
Contributions
Abstract: PB1637
Type: Publication Only
Background
Despite being the most common childhood cancer, nearly one half of ALL cases occurs in adults. Recently, it has been suggested that more intensive protocols may improve survival in adolescents and young adults (AYA).
Aims
Compare results of patients treated with BFM-based protocol to those patients treated with GMALL-based protocol, in a developing country.
Methods
This is a single center retrospective study which included all newly diagnosed adult ALL patients admitted between May/2012 and October/2016. Initially, patients aged 18-39 years (AYA group) were treated with BFM ALL 2009-based protocol and those aged 40-59 years were treated with GMALL 2003-based protocol. Since September 2013, because of high toxicity, only patients under 30 years were elegible for BFM-based treatment. Major adaptations were: (1) native E. coli l-asparaginase was substituted for peg-asparaginase, and (2) GMALL irradiation therapy was postponed to maintenance phase. BCR/ABL1 positive patients received standard chemotherapy plus Imatinib. Negative MRD was defined as < 0,01% by flow cytometry. Overall survival was estimated by Kaplan-Meier method. Competing risk analysis was carried out for cumulative incidence of death in CR1 or not in CR1. This study was approved by local Ethics Committee.
Results
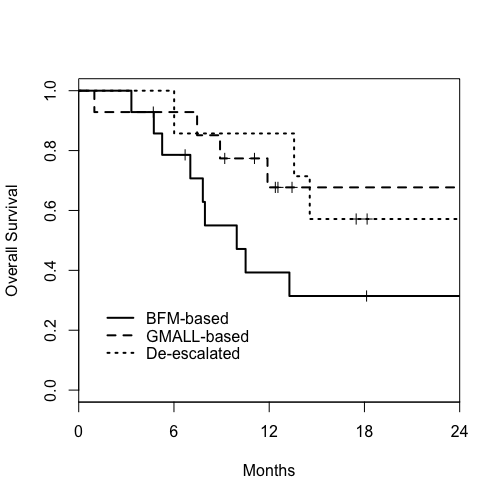
Thirty five patients were included, 21 of them started BFM-based treatment and 14 started GMALL-based protocol. During the first three months, 7 patients migrated from BFM to GMALL-based treatment because of toxicity and were analyzed separately. Median age was 21years (18-38) for BFM-based group, 44 years (30-57) for GMALL-based, and 33 years (21-38) for de-escalated. Male predominance was observed (71%), not different between groups. T-phenotype was more frequent than expected, representing 50% of BFM-based, 50% of GMALL-based and 29% of de-escalated groups. BCR/ABL1 was detected in 14% of BFM-based, 23% of GMALL-based and 14% of de-escalated groups (p = 0,85). Seven patients (2 BFM and 5 GMALL) underwent allogeneic stem cell transplantation in first remission. Of all 35 patients, 31 achieved complete remission after first induction phase. With median follow-up of 18 months, 1-year overall survival (OS) was 60% for all patients (39% for BFM-based, 75% for GMALL-based and 86% for de-escalated groups – p = 0,04; BFM-based versus other protocols). Cumulative incidence (CI) of death in first complete remission (CR1) at 12 months was 18%, not different between groups. CI of death at 12 months in non-CR1 (relapsed or refractory) patients was 39% for BFM-based, 7% for GMALL-based and 0% for de-escalated groups – BFM-based versus other HR 2,6; p 0,13. Among 31 patients who achieved CR1, MRD data was available for 26 (74%) of these at the end of first induction. OS at 18 months for CR1 patients with negative MDR after first induction was 74%, compared to 52% in MRD+.
Conclusion
Our results show that GMALL-based protocol yields good overall survival in adults ALL patients in a low income country, despite major adaptations. On the other hand, overall survival of AYA patients treated with BFM-based protocol was surprisingly poor, specially because of ineffective disease control. This finding may be related to several aspects: socioeconomic impairment, inadequate supportive care for more intense therapies and ineffective cancer care network. Future prospective studies should focus on this issues.
Session topic: 2. Acute lymphoblastic leukemia - Clinical
Keyword(s): Young adult, adult, Acute lymphoblastic leukemia
Abstract: PB1637
Type: Publication Only
Background
Despite being the most common childhood cancer, nearly one half of ALL cases occurs in adults. Recently, it has been suggested that more intensive protocols may improve survival in adolescents and young adults (AYA).
Aims
Compare results of patients treated with BFM-based protocol to those patients treated with GMALL-based protocol, in a developing country.
Methods
This is a single center retrospective study which included all newly diagnosed adult ALL patients admitted between May/2012 and October/2016. Initially, patients aged 18-39 years (AYA group) were treated with BFM ALL 2009-based protocol and those aged 40-59 years were treated with GMALL 2003-based protocol. Since September 2013, because of high toxicity, only patients under 30 years were elegible for BFM-based treatment. Major adaptations were: (1) native E. coli l-asparaginase was substituted for peg-asparaginase, and (2) GMALL irradiation therapy was postponed to maintenance phase. BCR/ABL1 positive patients received standard chemotherapy plus Imatinib. Negative MRD was defined as < 0,01% by flow cytometry. Overall survival was estimated by Kaplan-Meier method. Competing risk analysis was carried out for cumulative incidence of death in CR1 or not in CR1. This study was approved by local Ethics Committee.
Results
Thirty five patients were included, 21 of them started BFM-based treatment and 14 started GMALL-based protocol. During the first three months, 7 patients migrated from BFM to GMALL-based treatment because of toxicity and were analyzed separately. Median age was 21years (18-38) for BFM-based group, 44 years (30-57) for GMALL-based, and 33 years (21-38) for de-escalated. Male predominance was observed (71%), not different between groups. T-phenotype was more frequent than expected, representing 50% of BFM-based, 50% of GMALL-based and 29% of de-escalated groups. BCR/ABL1 was detected in 14% of BFM-based, 23% of GMALL-based and 14% of de-escalated groups (p = 0,85). Seven patients (2 BFM and 5 GMALL) underwent allogeneic stem cell transplantation in first remission. Of all 35 patients, 31 achieved complete remission after first induction phase. With median follow-up of 18 months, 1-year overall survival (OS) was 60% for all patients (39% for BFM-based, 75% for GMALL-based and 86% for de-escalated groups – p = 0,04; BFM-based versus other protocols). Cumulative incidence (CI) of death in first complete remission (CR1) at 12 months was 18%, not different between groups. CI of death at 12 months in non-CR1 (relapsed or refractory) patients was 39% for BFM-based, 7% for GMALL-based and 0% for de-escalated groups – BFM-based versus other HR 2,6; p 0,13. Among 31 patients who achieved CR1, MRD data was available for 26 (74%) of these at the end of first induction. OS at 18 months for CR1 patients with negative MDR after first induction was 74%, compared to 52% in MRD+.

Conclusion
Our results show that GMALL-based protocol yields good overall survival in adults ALL patients in a low income country, despite major adaptations. On the other hand, overall survival of AYA patients treated with BFM-based protocol was surprisingly poor, specially because of ineffective disease control. This finding may be related to several aspects: socioeconomic impairment, inadequate supportive care for more intense therapies and ineffective cancer care network. Future prospective studies should focus on this issues.
Session topic: 2. Acute lymphoblastic leukemia - Clinical
Keyword(s): Young adult, adult, Acute lymphoblastic leukemia


