THE FREQUENCY AND PROGNOSTIC SIGNIFICANCE OF IKZF1 DELETIONS IN ADULT PH-POSITIVE AND PH-NEGATIVE B-CELL ACUTE LYMPHOBLASTIC LEUKEMIA PATIENTS TREATED IN RUSSIAN ACUTE LYMPHOBLASTIC LEUKEMIA STUDIES.
(Abstract release date: 05/18/17)
EHA Library. Isinova G. 05/18/17; 182350; PB1636

Galina Isinova
Contributions
Contributions
Abstract
Abstract: PB1636
Type: Publication Only
Background
The incidence of IKZF1 gene deletions is approximately 20 % in adult patients with BCR-ABL1- negative B-cell ALL and 70–80% in BCR-ABL1-positive ALL. These mutations are associated with poor prognosis in patients with Ph-negative ALL, but not in patients with Ph-positive ALL, suggesting that IKZF1 deletions may be more prognostically valuable in patients with Ph-negative ALL.
Aims
To evaluate the frequency and prognostic impact of mutation status of IKZF1 in patients with de novo BCR-ABL1-negative and BCR-ABL1-positive B-cell acute lymphoblastic leukemia.
Methods
The study included 36 adult patients (median age 27, range 17-56; m:f=15:21) with newly diagnosed BCR-ABL1- neg B-cell ALL and 15 patients (median age 34 years, range 22–68; m:f=6:9) with BCR-ABL1- pos B-cell ALL, who were enrolled in Russian acute lymphoblastic leukemia (RALL) - 2009 [ClinicalTrials.gov public site; NCT01193933] and RALL-2012 protocols since Feb 2010 till Sep 2016 and Aug 2009 till Feb 2017, respectively.
Intragenic deletions of IKZF1 were detected using breakpoint-specific fluorescent multiplex polymerase chain reaction according to the procedure described by [Aurelie Caye et al, Haematologica, 2013]. DNA for PCR was extracted from leukemia cells of frozen bone marrow samples.
Results
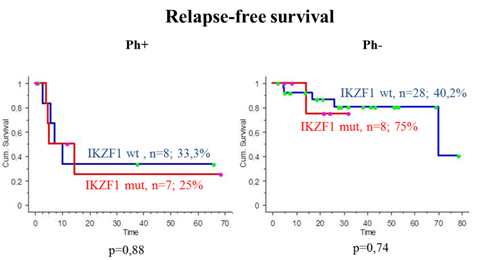
The IKZF1 deletions were detected in 7 (47%) of 15 patients with BCR-ABL1- pos ALL (3 cases with del 4-7 (43%), 2 – del 2-7 (28%), 1 – del 2a-8 and 1 – del 4-8 (14%)). The median follow-up time in 15 patients was 18 months (range: 4-79 month). Five patients died (33%) after relapse or progression of the disease, and 10 patients are alive. Overall survival (OS) in BCR-ABL1 - pos B-cell ALL patients with IKZF1 mutations and without was 37,5 % and 57,1 % (p=0,77), relapse - free survival (RFS) - 25% and 33,3% (p=0,88), respectively.
In patients with BCR-ABL1- neg ALL the IKZF1 deletions were revealed in 8 (22%) of 36 patients (4 cases with del 4-7 (50%), 2 - del 2-7 (25 %), 1 – del of 2-8 (12,5%) and in 1 patient all types of deletions were determined (del 4-7, del 4-8, del 2-7, del 2-8)). The median follow-up time in 36 patients was 22 months (range: 0,5-84 month). 4 patients died of the disease (11%) and 2 of infections, 30 patients are alive. OS for patients with BCR-ABL1- neg ALL with IKZF1 mutations and without was 100% and 60,2% (p=0,77), RFS - 75% and 40,2% (р=0,74), respectively. (Fig.1).
Conclusion
The frequency of IKZF1 gene deletions in patients with BCR-ABL1- pos and with BCR-ABL1- neg ALL was 47% and 22%, respectively. IKZF1 mutations seemed to be of poor prognosis for BCR-ABL1-pos ALL and, on the contrary, more favorable for BCR-ABL1- neg ALL, though not statistically significant. Having or not IKZF1 mutations, all BCR-ABL1- pos ALL patients are candidates for allogeneic hematopoietic stem cell transplantation (allo-HSCT). Regarding BCR-ABL1-neg ALL: though the group of patients is small, we can suggest that IKZF1 mutation did not appear to influence survival due to different chemotherapy principal in RALL – 2009 – non-intensive but not-interruptive therapy with low numbers of HSCT.
Session topic: 2. Acute lymphoblastic leukemia - Clinical
Keyword(s): Ikaros, ALL, adult
Abstract: PB1636
Type: Publication Only
Background
The incidence of IKZF1 gene deletions is approximately 20 % in adult patients with BCR-ABL1- negative B-cell ALL and 70–80% in BCR-ABL1-positive ALL. These mutations are associated with poor prognosis in patients with Ph-negative ALL, but not in patients with Ph-positive ALL, suggesting that IKZF1 deletions may be more prognostically valuable in patients with Ph-negative ALL.
Aims
To evaluate the frequency and prognostic impact of mutation status of IKZF1 in patients with de novo BCR-ABL1-negative and BCR-ABL1-positive B-cell acute lymphoblastic leukemia.
Methods
The study included 36 adult patients (median age 27, range 17-56; m:f=15:21) with newly diagnosed BCR-ABL1- neg B-cell ALL and 15 patients (median age 34 years, range 22–68; m:f=6:9) with BCR-ABL1- pos B-cell ALL, who were enrolled in Russian acute lymphoblastic leukemia (RALL) - 2009 [ClinicalTrials.gov public site; NCT01193933] and RALL-2012 protocols since Feb 2010 till Sep 2016 and Aug 2009 till Feb 2017, respectively.
Intragenic deletions of IKZF1 were detected using breakpoint-specific fluorescent multiplex polymerase chain reaction according to the procedure described by [Aurelie Caye et al, Haematologica, 2013]. DNA for PCR was extracted from leukemia cells of frozen bone marrow samples.
Results
The IKZF1 deletions were detected in 7 (47%) of 15 patients with BCR-ABL1- pos ALL (3 cases with del 4-7 (43%), 2 – del 2-7 (28%), 1 – del 2a-8 and 1 – del 4-8 (14%)). The median follow-up time in 15 patients was 18 months (range: 4-79 month). Five patients died (33%) after relapse or progression of the disease, and 10 patients are alive. Overall survival (OS) in BCR-ABL1 - pos B-cell ALL patients with IKZF1 mutations and without was 37,5 % and 57,1 % (p=0,77), relapse - free survival (RFS) - 25% and 33,3% (p=0,88), respectively.
In patients with BCR-ABL1- neg ALL the IKZF1 deletions were revealed in 8 (22%) of 36 patients (4 cases with del 4-7 (50%), 2 - del 2-7 (25 %), 1 – del of 2-8 (12,5%) and in 1 patient all types of deletions were determined (del 4-7, del 4-8, del 2-7, del 2-8)). The median follow-up time in 36 patients was 22 months (range: 0,5-84 month). 4 patients died of the disease (11%) and 2 of infections, 30 patients are alive. OS for patients with BCR-ABL1- neg ALL with IKZF1 mutations and without was 100% and 60,2% (p=0,77), RFS - 75% and 40,2% (р=0,74), respectively. (Fig.1).

Conclusion
The frequency of IKZF1 gene deletions in patients with BCR-ABL1- pos and with BCR-ABL1- neg ALL was 47% and 22%, respectively. IKZF1 mutations seemed to be of poor prognosis for BCR-ABL1-pos ALL and, on the contrary, more favorable for BCR-ABL1- neg ALL, though not statistically significant. Having or not IKZF1 mutations, all BCR-ABL1- pos ALL patients are candidates for allogeneic hematopoietic stem cell transplantation (allo-HSCT). Regarding BCR-ABL1-neg ALL: though the group of patients is small, we can suggest that IKZF1 mutation did not appear to influence survival due to different chemotherapy principal in RALL – 2009 – non-intensive but not-interruptive therapy with low numbers of HSCT.
Session topic: 2. Acute lymphoblastic leukemia - Clinical
Keyword(s): Ikaros, ALL, adult
{{ help_message }}
{{filter}}


