A COMPREHENSIVE ANALYSIS OF PATIENT- AND THERAPY-RELATED FACTORS AFFECTING THE TOXICITY OF PEGYLATED-ASPARAGINASE FOR THE TREATMENT OF ADULT ACUTE LYMPHOBLASTIC LEUKEMIA
(Abstract release date: 05/18/17)
EHA Library. Minetto P. 05/18/17; 182346; PB1632

Dr. Paola Minetto
Contributions
Contributions
Abstract
Abstract: PB1632
Type: Publication Only
Background
The application of pediatric regimens in the treatment of adult acute lymphoblastic leukemia (ALL) has led to a significant improvement in patients outcome. However, concerns about the feasibility of more intensive therapies and of the use of pegylated L-Asparaginase (PEG-ASP) in adult patients have emerged. Some patient-related risk factors as high BMI or hepatic steatosis have been already identified as risk factors, but few data are available on the synergic toxic effect from other concomitant drugs.
Aims
The aim of the present study was to evaluate the incidence of PEG-ASP related adverse events in a cohort of adult ALL patients in order to identify potential patient and therapy-related risk factors contributing to toxicity.
Methods
Since 2013, 21 adult ALL patients received PEG-ASP therapy in our institution. Median age was 44 (range 19-76); 12 patients were treated in frontline setting (7 according to a full pediatric protocol) whereas 9 patients received therapy for relapsed/refractory neoplasm.
We retrospectively analyzed each single course which included PEG-ASP administration as an independent event, accounting 41 episodes. Patients’ features (age, BMI, disease status) and concomitant therapies were accurately analyzed as factors potentially affecting PEG-ASP toxicity. The incidence of major thrombotic/bleeding complications and grade III/IV hepatic or pancreatic toxicity was analyzed; toxicity grading and management of PEG-ASP related complications were performed according to guidelines recently published by Stock et al.
Results
No grade III/IV pancreatic, thrombotic or hemorrhagic adverse events were recorded.
A total of 8 episodes of grade III/IV hepatic toxicities were observed. In 3 cases, grade IV toxicity was observed. Those patients experienced unexplained severe weight gain and painful epathomegaly, a clinical picture resembling sinusoidal occlusive disease, ultrasonography showed acute liver steatosis. All 3 patients received concomitant therapy with idarubicin, vincristine and vancomycin.
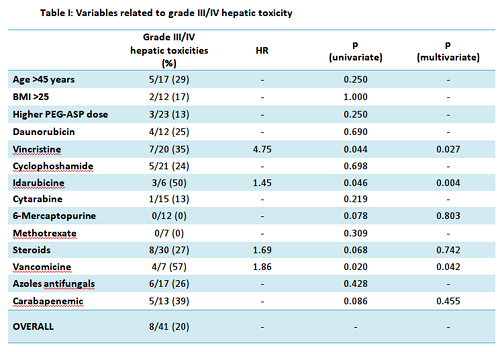
In univariate analysis, the incidence of grade III/IV hepatic toxicity was significantly higher when concomitant chemotherapy with at least 2 mg/sqm cumulative dose of vincristine (p 0.044, HR 4.75) or at least 16 mg/sqm cumulative dose of idarubicine (p 0.046, HR 1.45) were administered. Steroids therapy determined a borderline increase in toxicity risk (p 0.068, HR 1.688). No increase in toxicity was observed with any dosing of daunorubicin, cyclophosphamide, cytarabine, methotrexate and 6-mercaptopurine (Tab. I).
Among concomitant antibiotic therapies, vancomycin administration seemed to increase the incidence of grade III/IV hepato-toxicy (p 0.02, HR 1.863). No significant increase was observed with carbapenems and azoles (Tab I).
Older age (>45), receiving PEG-ASP with active leukemia or a high BMI (>25) were not related with an increased incidence of grade III/IV hepato-toxicity (Tab I). Notably, none of the patients undergoing full pediatric induction (who received the highest doses of PEG-ASP), regardless of age (ranging from 21 to 55) experienced grade III/IV hepatotoxicity.
A multivariate logistic regression analysis disclosed that concomitant administration of idarubicin, vincristine or vancomycin were independent predictors of grade III/IV hepatotoxicity (p 0.004, 0.027 and 0.042, respectively, Tab. I).
Conclusion
Our data show that the toxicity profile of PEG-ASP in adult patients is overall manageable. However, serious warnings emerge from our experience. Concomitant drugs and their timing of administration may play a crucial role in significantly contributing to PEG-ASP hepatic toxicity. In order to attempt to reduce toxicity, anthracyclines with shorter half-life, i.e. daunorubicin instead of idarubicin, should be used. A particular attention should be paid when administration of concomitant antibiotic therapy is required.
Session topic: 2. Acute lymphoblastic leukemia - Clinical
Keyword(s): Acute lymphoblastic leukemia, Veno-occlusive disease, toxicity, Asparaginase
Abstract: PB1632
Type: Publication Only
Background
The application of pediatric regimens in the treatment of adult acute lymphoblastic leukemia (ALL) has led to a significant improvement in patients outcome. However, concerns about the feasibility of more intensive therapies and of the use of pegylated L-Asparaginase (PEG-ASP) in adult patients have emerged. Some patient-related risk factors as high BMI or hepatic steatosis have been already identified as risk factors, but few data are available on the synergic toxic effect from other concomitant drugs.
Aims
The aim of the present study was to evaluate the incidence of PEG-ASP related adverse events in a cohort of adult ALL patients in order to identify potential patient and therapy-related risk factors contributing to toxicity.
Methods
Since 2013, 21 adult ALL patients received PEG-ASP therapy in our institution. Median age was 44 (range 19-76); 12 patients were treated in frontline setting (7 according to a full pediatric protocol) whereas 9 patients received therapy for relapsed/refractory neoplasm.
We retrospectively analyzed each single course which included PEG-ASP administration as an independent event, accounting 41 episodes. Patients’ features (age, BMI, disease status) and concomitant therapies were accurately analyzed as factors potentially affecting PEG-ASP toxicity. The incidence of major thrombotic/bleeding complications and grade III/IV hepatic or pancreatic toxicity was analyzed; toxicity grading and management of PEG-ASP related complications were performed according to guidelines recently published by Stock et al.
Results
No grade III/IV pancreatic, thrombotic or hemorrhagic adverse events were recorded.
A total of 8 episodes of grade III/IV hepatic toxicities were observed. In 3 cases, grade IV toxicity was observed. Those patients experienced unexplained severe weight gain and painful epathomegaly, a clinical picture resembling sinusoidal occlusive disease, ultrasonography showed acute liver steatosis. All 3 patients received concomitant therapy with idarubicin, vincristine and vancomycin.
In univariate analysis, the incidence of grade III/IV hepatic toxicity was significantly higher when concomitant chemotherapy with at least 2 mg/sqm cumulative dose of vincristine (p 0.044, HR 4.75) or at least 16 mg/sqm cumulative dose of idarubicine (p 0.046, HR 1.45) were administered. Steroids therapy determined a borderline increase in toxicity risk (p 0.068, HR 1.688). No increase in toxicity was observed with any dosing of daunorubicin, cyclophosphamide, cytarabine, methotrexate and 6-mercaptopurine (Tab. I).
Among concomitant antibiotic therapies, vancomycin administration seemed to increase the incidence of grade III/IV hepato-toxicy (p 0.02, HR 1.863). No significant increase was observed with carbapenems and azoles (Tab I).
Older age (>45), receiving PEG-ASP with active leukemia or a high BMI (>25) were not related with an increased incidence of grade III/IV hepato-toxicity (Tab I). Notably, none of the patients undergoing full pediatric induction (who received the highest doses of PEG-ASP), regardless of age (ranging from 21 to 55) experienced grade III/IV hepatotoxicity.
A multivariate logistic regression analysis disclosed that concomitant administration of idarubicin, vincristine or vancomycin were independent predictors of grade III/IV hepatotoxicity (p 0.004, 0.027 and 0.042, respectively, Tab. I).

Conclusion
Our data show that the toxicity profile of PEG-ASP in adult patients is overall manageable. However, serious warnings emerge from our experience. Concomitant drugs and their timing of administration may play a crucial role in significantly contributing to PEG-ASP hepatic toxicity. In order to attempt to reduce toxicity, anthracyclines with shorter half-life, i.e. daunorubicin instead of idarubicin, should be used. A particular attention should be paid when administration of concomitant antibiotic therapy is required.
Session topic: 2. Acute lymphoblastic leukemia - Clinical
Keyword(s): Acute lymphoblastic leukemia, Veno-occlusive disease, toxicity, Asparaginase
{{ help_message }}
{{filter}}


