
Contributions
Abstract: S807
Type: Oral Presentation
Presentation during EHA22: On Sunday, June 25, 2017 from 08:45 - 09:00
Location: Room N104
Background
CMV remains a common complication of HCT, yet no antiviral drug suitable for prophylaxis is available in HCT. LET is a first-in-class drug that inhibits the CMV terminase complex. A dose-escalation phase 2 trial showed that LET prophylaxis for up to 12 weeks post-HCT was effective with a safety profile similar to placebo.
Aims
To compare LET prophylaxis to placebo for the prevention of clinically significant CMV infection (CS-CMV), defined as CMV disease or CMV viremia leading to preemptive treatment (PET) in a Phase III randomized, double-blind, placebo-controlled trial.
Methods
CMV seropositive HCT recipients 18 years or older who had undetectable plasma CMV DNA within 5 days of randomization were eligible (full eligibility at clinicaltrials.gov, NCT02137772). Subjects had to start treatment by Day+28 post-HCT. Subjects were randomized 2:1 to receive LET or placebo PO or IV through Week 14 (Day +100) post-HCT, stratified by study site and high or low CMV disease risk. LET was dosed at 480 mg/d (or 240 mg/d if on cyclosporine due to drug-drug interaction). Subjects were assessed weekly through Week 14, biweekly through Week 24, and every other month through Week 48 after HCT. Plasma obtained at each visit was assayed for CMV DNA in a central laboratory. Subjects who developed CS-CMV discontinued study drug and received anti- CMV treatment. Local CMV assay results could be used to start PET. The primary endpoint was the stratum-adjusted proportion of subjects with CS-CMV through Week 24 post-HCT among subjects with undetectable CMV DNA at randomization; subjects who discontinued the study for any reason or with missing data at Week 24 were considered failures. All adverse events (AEs) were analyzed through 14 days after the last dose of study drug.
Results
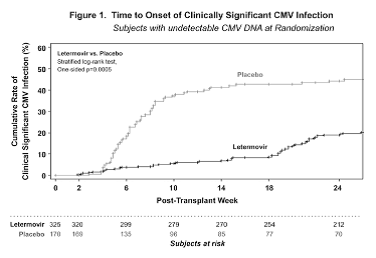
From June 2014 to March 2016, 565 randomized subjects received study treatment; 31% were at high CMV disease risk. 50% subjects received myeloablative conditioning, 35% received ATG. Donors included 14% mismatched unrelated, 13% haploidentical and 4% cord blood. Study arms were balanced. Subjects began study drug a median of 9 days post-HCT; 37% had engrafted prior to start. Of 495 treated subjects with undetectable CMV DNA at randomization, fewer subjects developed CS-CMV or were considered failures in the LET arm (122/325, 38%) compared to placebo (103/170, 61%; p<0.0001) by Week 24 post-HCT. Figure 1 shows the time to CS-CMV analysis. The most common AEs (LET, placebo) were GVHD (39%, 39%), diarrhea (26%, 25%), and nausea (27%, 23%). More frequent vomiting (19%, 14%), edema (15%, 9%), atrial arrhythmias (10%, 5%), and ALT levels > 5xULN (4%, 2%) was noted in LET-treated subjects; no increased myelotoxicity or nephrotoxicity was observed. The Week 24 all-cause mortality was 10% for LET recipients and 15% for placebo recipients.
Conclusion
Letermovir prophylaxis was effective in reducing clinically significant CMV infection, was overall well tolerated, and provides a new approach to CMV prevention after HCT.
Session topic: 29. Infectious diseases, supportive care
Keyword(s): Prophylaxis, CMV, Allogeneic hematopoietic stem cell transplant
Abstract: S807
Type: Oral Presentation
Presentation during EHA22: On Sunday, June 25, 2017 from 08:45 - 09:00
Location: Room N104
Background
CMV remains a common complication of HCT, yet no antiviral drug suitable for prophylaxis is available in HCT. LET is a first-in-class drug that inhibits the CMV terminase complex. A dose-escalation phase 2 trial showed that LET prophylaxis for up to 12 weeks post-HCT was effective with a safety profile similar to placebo.
Aims
To compare LET prophylaxis to placebo for the prevention of clinically significant CMV infection (CS-CMV), defined as CMV disease or CMV viremia leading to preemptive treatment (PET) in a Phase III randomized, double-blind, placebo-controlled trial.
Methods
CMV seropositive HCT recipients 18 years or older who had undetectable plasma CMV DNA within 5 days of randomization were eligible (full eligibility at clinicaltrials.gov, NCT02137772). Subjects had to start treatment by Day+28 post-HCT. Subjects were randomized 2:1 to receive LET or placebo PO or IV through Week 14 (Day +100) post-HCT, stratified by study site and high or low CMV disease risk. LET was dosed at 480 mg/d (or 240 mg/d if on cyclosporine due to drug-drug interaction). Subjects were assessed weekly through Week 14, biweekly through Week 24, and every other month through Week 48 after HCT. Plasma obtained at each visit was assayed for CMV DNA in a central laboratory. Subjects who developed CS-CMV discontinued study drug and received anti- CMV treatment. Local CMV assay results could be used to start PET. The primary endpoint was the stratum-adjusted proportion of subjects with CS-CMV through Week 24 post-HCT among subjects with undetectable CMV DNA at randomization; subjects who discontinued the study for any reason or with missing data at Week 24 were considered failures. All adverse events (AEs) were analyzed through 14 days after the last dose of study drug.
Results
From June 2014 to March 2016, 565 randomized subjects received study treatment; 31% were at high CMV disease risk. 50% subjects received myeloablative conditioning, 35% received ATG. Donors included 14% mismatched unrelated, 13% haploidentical and 4% cord blood. Study arms were balanced. Subjects began study drug a median of 9 days post-HCT; 37% had engrafted prior to start. Of 495 treated subjects with undetectable CMV DNA at randomization, fewer subjects developed CS-CMV or were considered failures in the LET arm (122/325, 38%) compared to placebo (103/170, 61%; p<0.0001) by Week 24 post-HCT. Figure 1 shows the time to CS-CMV analysis. The most common AEs (LET, placebo) were GVHD (39%, 39%), diarrhea (26%, 25%), and nausea (27%, 23%). More frequent vomiting (19%, 14%), edema (15%, 9%), atrial arrhythmias (10%, 5%), and ALT levels > 5xULN (4%, 2%) was noted in LET-treated subjects; no increased myelotoxicity or nephrotoxicity was observed. The Week 24 all-cause mortality was 10% for LET recipients and 15% for placebo recipients.

Conclusion
Letermovir prophylaxis was effective in reducing clinically significant CMV infection, was overall well tolerated, and provides a new approach to CMV prevention after HCT.
Session topic: 29. Infectious diseases, supportive care
Keyword(s): Prophylaxis, CMV, Allogeneic hematopoietic stem cell transplant


