DISCONTINUING ANTIBACTERIAL THERAPY AFTER APYREXIA AND CLINICAL STABILITY REGARDLESS OF NEUTROPHIL COUNT IN FEBRIL NEUTROPENIA IS SAFE AND REDUCES EXPOSITION TO ANTIBIOTICS (HOWLONG RANDOMIZED TRIAL)
(Abstract release date: 05/18/17)
EHA Library. Espigado I. 06/25/17; 182091; S804

Ildefonso Espigado
Contributions
Contributions
Abstract
Abstract: S804
Type: Oral Presentation
Presentation during EHA22: On Sunday, June 25, 2017 from 08:00 - 08:15
Location: Room N104
Background
In neutropenic patients with unexplained fever the classical approach is maintaining the empirical antibacterial therapy (EAT) until neutrophil recovery. This strategy may result in unnecessarily prolonged EAT favoring bacterial resistance, organ toxicity and damage to microbiota. Nevertheless, the available scientific evidence supporting the alternative approach of stopping EAT before neutrofile recovery is moderate.
Aims
To investigate if a clinical approach (based on apyrexia and clinical recovery) is better than and as safe as the standard criteria (recovery from neutropenia) to decide the discontinuation of EAT.
Methods
After local Ethical Committee approval, a randomized, controlled, multicenter, open-labeled phase IV clinical trial was performed (EudraCT: 2011-005152-34). Study period: May-2012 to May-2016. Inclusion criteria: a) Adult patients (≥18 years); b) Hematologic malignancy or autologous or allogeneic hematopoietic stem cell transplantation (SCT) recipients; c) High risk febrile neutropenia (FN) d) Informed consent signed. Exclusion criteria: etiological diagnosis of FN. Patients were randomized 72 hours after fever onset to: 1. Experimental group (EG): EAT discontinuation if a) apyrexia ≥ 72 h, plus b) clinical recovery ≥ 72 h (independently of neutrophil count) or 2. Control group (CG): EAT discontinuation if a) apyrexia ≥ 72 h, plus b) clinical recovery ≥ 72 h, plus c) >0.5x106/L neutrophils. Follow-up: 28 days from EAT. Primary (efficacy) end-point was number of EAT-free days. Secondary (safety) end-points were total days of fever and crude mortality.
Results
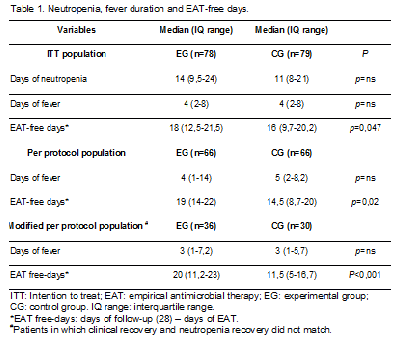
One hundred and fifty seven patients were included (EG 78 and CG 79). There were no differences in baseline characteristics or clinical presentation between groups. The most frequent underlying conditions were induction/re-induction chemotherapy for acute leukemia (n=42, 26,7%), autologous SCT (n=42, 45,8%), and allogeneic SCT (n=14, 8,9%). The most frequent clinical presentation was non-focused FN (n=63, 40,1%), abdominal focused FN (n=34, 21,6%) and mucositis (n=31, 19,7%). Days with fever, and neutropenia duration and EAT-free days difference between groups are detailed in Table 1. Recurrent fever frequency was 14,3% (EG) and 17,9% (CG) (p=ns) and crude mortality was 1,3% (EG) and 3,8% (CG) (p=ns).
Conclusion
In hematological patients with febrile neutropenia of unknown origin the discontinuation of empirical antibacterial therapy after 72 hours of apyrexia and clinical recovery regardless of neutrophils count is safe and reduces unnecessary exposure to antibiotics.
Session topic: 29. Infectious diseases, supportive care
Keyword(s): neutropenia, Infection, Febrile neutropenia, Clinical Trial
Abstract: S804
Type: Oral Presentation
Presentation during EHA22: On Sunday, June 25, 2017 from 08:00 - 08:15
Location: Room N104
Background
In neutropenic patients with unexplained fever the classical approach is maintaining the empirical antibacterial therapy (EAT) until neutrophil recovery. This strategy may result in unnecessarily prolonged EAT favoring bacterial resistance, organ toxicity and damage to microbiota. Nevertheless, the available scientific evidence supporting the alternative approach of stopping EAT before neutrofile recovery is moderate.
Aims
To investigate if a clinical approach (based on apyrexia and clinical recovery) is better than and as safe as the standard criteria (recovery from neutropenia) to decide the discontinuation of EAT.
Methods
After local Ethical Committee approval, a randomized, controlled, multicenter, open-labeled phase IV clinical trial was performed (EudraCT: 2011-005152-34). Study period: May-2012 to May-2016. Inclusion criteria: a) Adult patients (≥18 years); b) Hematologic malignancy or autologous or allogeneic hematopoietic stem cell transplantation (SCT) recipients; c) High risk febrile neutropenia (FN) d) Informed consent signed. Exclusion criteria: etiological diagnosis of FN. Patients were randomized 72 hours after fever onset to: 1. Experimental group (EG): EAT discontinuation if a) apyrexia ≥ 72 h, plus b) clinical recovery ≥ 72 h (independently of neutrophil count) or 2. Control group (CG): EAT discontinuation if a) apyrexia ≥ 72 h, plus b) clinical recovery ≥ 72 h, plus c) >0.5x106/L neutrophils. Follow-up: 28 days from EAT. Primary (efficacy) end-point was number of EAT-free days. Secondary (safety) end-points were total days of fever and crude mortality.
Results
One hundred and fifty seven patients were included (EG 78 and CG 79). There were no differences in baseline characteristics or clinical presentation between groups. The most frequent underlying conditions were induction/re-induction chemotherapy for acute leukemia (n=42, 26,7%), autologous SCT (n=42, 45,8%), and allogeneic SCT (n=14, 8,9%). The most frequent clinical presentation was non-focused FN (n=63, 40,1%), abdominal focused FN (n=34, 21,6%) and mucositis (n=31, 19,7%). Days with fever, and neutropenia duration and EAT-free days difference between groups are detailed in Table 1. Recurrent fever frequency was 14,3% (EG) and 17,9% (CG) (p=ns) and crude mortality was 1,3% (EG) and 3,8% (CG) (p=ns).

Conclusion
In hematological patients with febrile neutropenia of unknown origin the discontinuation of empirical antibacterial therapy after 72 hours of apyrexia and clinical recovery regardless of neutrophils count is safe and reduces unnecessary exposure to antibiotics.
Session topic: 29. Infectious diseases, supportive care
Keyword(s): neutropenia, Infection, Febrile neutropenia, Clinical Trial
{{ help_message }}
{{filter}}


