
Contributions
Abstract: S792
Type: Oral Presentation
Presentation during EHA22: On Sunday, June 25, 2017 from 08:45 - 09:00
Location: Room N101
Background
The fms-like tyrosine kinase 3 internal tandem duplication (FLT3-ITD) mutation is a genetic alteration found in approximately 30% of patients with acute myeloid leukemia (AML). Although patients with FLT3-ITD AML achieve remission rates similar to those with FLT3 wildtype status with induction chemotherapy regimens; patients with FLT3-ITD have significantly shorter remission durations and increased rates of relapse. Even though allogeneic SCT improves outcomes, patients still have higher rates of relapse comparatively with poor prognosis post relapse. Sorafenib(SFB) is a TKI with activity against RAF, VEGF and FLT3-ITD and its use as maintenance therapy after allogeneic SCT has been shown as a promising approach to decrease relapse. Several studies report that SFB maintenance post SCT provides durable complete responses; however, there are also descriptions of sorafenib post SCT triggering acute GVHD, cytopenias, rash and diarrhea.
Aims
To assess the outcomes, including progression free survival (PFS) and overall survival (OS), in patients with FLT3-ITD mutated AML who receive SFB maintenance after allogeneic SCT.
Methods
We analyzed adult patients (age≥18) with a diagnosis of FLT3-ITD mutated AML who received an allogeneic SCT between 1/1/2010 and 10/28/16 at our institution. Using a case control analysis and matching patients who received maintenance SFB (maintenance group) with control patients, FLT3-ITD mutated AML who did not receive maintenance post SCT(control group); we matched each case to two control patients accounting for disease status, type of conditioning, donor type, cytogenetic risk factors and age. To be considered as maintenance, SFB had to be started within 101 days of the SCT. To reduce bias from disease risks and transplant-related mortality (TRM), all patients were required to be in complete remission (CR) at study entry - defined as the date of SFB initiation for cases and the same time point after SCT for their matched controls without maintenance.
Results
Among the 214 AML patients with FLT3-ITD mutation that underwent SCT during study period, we identified 13 cases (maintenance) and 26 controls (no maintenance). Median follow-up of survivors were 12 months and 30 months for maintenance and control group respectively.
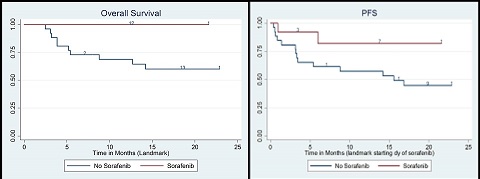
Conclusion
Sorafenib maintenance is safe and can produce long term durable remissions after allogeneic stem cell transplant in a high risk population with FLT3-ITD mutated AML.
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): Flt3 inhibitor, AML, Allogeneic hematopoietic stem cell transplant, Flt3-ITD
Abstract: S792
Type: Oral Presentation
Presentation during EHA22: On Sunday, June 25, 2017 from 08:45 - 09:00
Location: Room N101
Background
The fms-like tyrosine kinase 3 internal tandem duplication (FLT3-ITD) mutation is a genetic alteration found in approximately 30% of patients with acute myeloid leukemia (AML). Although patients with FLT3-ITD AML achieve remission rates similar to those with FLT3 wildtype status with induction chemotherapy regimens; patients with FLT3-ITD have significantly shorter remission durations and increased rates of relapse. Even though allogeneic SCT improves outcomes, patients still have higher rates of relapse comparatively with poor prognosis post relapse. Sorafenib(SFB) is a TKI with activity against RAF, VEGF and FLT3-ITD and its use as maintenance therapy after allogeneic SCT has been shown as a promising approach to decrease relapse. Several studies report that SFB maintenance post SCT provides durable complete responses; however, there are also descriptions of sorafenib post SCT triggering acute GVHD, cytopenias, rash and diarrhea.
Aims
To assess the outcomes, including progression free survival (PFS) and overall survival (OS), in patients with FLT3-ITD mutated AML who receive SFB maintenance after allogeneic SCT.
Methods
We analyzed adult patients (age≥18) with a diagnosis of FLT3-ITD mutated AML who received an allogeneic SCT between 1/1/2010 and 10/28/16 at our institution. Using a case control analysis and matching patients who received maintenance SFB (maintenance group) with control patients, FLT3-ITD mutated AML who did not receive maintenance post SCT(control group); we matched each case to two control patients accounting for disease status, type of conditioning, donor type, cytogenetic risk factors and age. To be considered as maintenance, SFB had to be started within 101 days of the SCT. To reduce bias from disease risks and transplant-related mortality (TRM), all patients were required to be in complete remission (CR) at study entry - defined as the date of SFB initiation for cases and the same time point after SCT for their matched controls without maintenance.
Results
Among the 214 AML patients with FLT3-ITD mutation that underwent SCT during study period, we identified 13 cases (maintenance) and 26 controls (no maintenance). Median follow-up of survivors were 12 months and 30 months for maintenance and control group respectively.

Conclusion
Sorafenib maintenance is safe and can produce long term durable remissions after allogeneic stem cell transplant in a high risk population with FLT3-ITD mutated AML.
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): Flt3 inhibitor, AML, Allogeneic hematopoietic stem cell transplant, Flt3-ITD


