
Contributions
Abstract: S782
Type: Oral Presentation
Presentation during EHA22: On Sunday, June 25, 2017 from 08:45 - 09:00
Location: Hall D
Background
Multiple myeloma is characterized by osteolytic bone disease, with up to 80% of pts presenting with detectable lesions. Myeloma bone disease is mediated by osteoclast activating factors such as RANKL, increasing the risk of skeletal-related events (SREs) and impacting morbidity and mortality. DMB, a human monoclonal antibody that targets and binds to RANKL, can be administered subcutaneously (SC) to pts regardless of renal function.
Aims
This study evaluates the efficacy and safety of DMB compared with ZA in newly diagnosed myeloma pts.
Methods
Adult pts were randomized 1:1 to DMB 120mg SC Q4W or ZA 4mg IV (adjusted) Q4W along with anti-myeloma therapy. Key stratification factors included type of first-line therapy (novel or non-novel) and previous SRE. Pts with renal insufficiency were excluded if baseline creatinine clearance (CrCl)<30mL/min. The primary endpoint was non-inferiority of DMB to ZA with respect to time to first on-study SRE. Secondary endpoints included superiority of DMB for time to first on-study SRE and first-and-subsequent on-study SRE, and overall survival (OS). Progression-free survival (PFS) was an exploratory endpoint. Safety was also assessed.
Results
A total of 1718 pts were randomized, 859 to each arm. Baseline demographics and disease characteristics were balanced, with 66.0% of DMB and 67.2% of ZA pts reporting prior SRE history; CrCl≤60mL/min was reported in 26.7% of pts. During the primary blinded treatment period (median follow-up 17.4 months [m]), 43.8% DMB pts and 44.6% ZA pts had a first on-study SRE. The median time to first on-study SRE was similar between DMB (22.83 m) and ZA (23.98 m) pts.
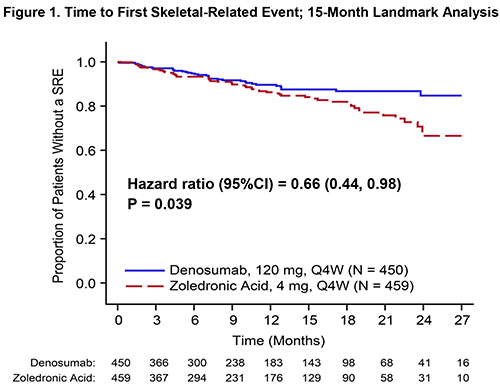
Conclusion
DMB demonstrated non-inferiority to ZA in delaying time to first on-study SRE in myeloma pts, meeting the primary endpoint of the study. A landmark analysis at 15 m suggests a significant benefit for DMB with respect to time to first SRE. The rates of renal AEs were significantly lower in DMB pts while the overall rates of AEs, including hypocalcemia and ONJ, were consistent with the known DMB safety profile. The results of the landmark analysis and possible prolongation of PFS with DMB therapy is promising.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Multiple Myeloma, Bone disease
Abstract: S782
Type: Oral Presentation
Presentation during EHA22: On Sunday, June 25, 2017 from 08:45 - 09:00
Location: Hall D
Background
Multiple myeloma is characterized by osteolytic bone disease, with up to 80% of pts presenting with detectable lesions. Myeloma bone disease is mediated by osteoclast activating factors such as RANKL, increasing the risk of skeletal-related events (SREs) and impacting morbidity and mortality. DMB, a human monoclonal antibody that targets and binds to RANKL, can be administered subcutaneously (SC) to pts regardless of renal function.
Aims
This study evaluates the efficacy and safety of DMB compared with ZA in newly diagnosed myeloma pts.
Methods
Adult pts were randomized 1:1 to DMB 120mg SC Q4W or ZA 4mg IV (adjusted) Q4W along with anti-myeloma therapy. Key stratification factors included type of first-line therapy (novel or non-novel) and previous SRE. Pts with renal insufficiency were excluded if baseline creatinine clearance (CrCl)<30mL/min. The primary endpoint was non-inferiority of DMB to ZA with respect to time to first on-study SRE. Secondary endpoints included superiority of DMB for time to first on-study SRE and first-and-subsequent on-study SRE, and overall survival (OS). Progression-free survival (PFS) was an exploratory endpoint. Safety was also assessed.
Results
A total of 1718 pts were randomized, 859 to each arm. Baseline demographics and disease characteristics were balanced, with 66.0% of DMB and 67.2% of ZA pts reporting prior SRE history; CrCl≤60mL/min was reported in 26.7% of pts. During the primary blinded treatment period (median follow-up 17.4 months [m]), 43.8% DMB pts and 44.6% ZA pts had a first on-study SRE. The median time to first on-study SRE was similar between DMB (22.83 m) and ZA (23.98 m) pts.

Conclusion
DMB demonstrated non-inferiority to ZA in delaying time to first on-study SRE in myeloma pts, meeting the primary endpoint of the study. A landmark analysis at 15 m suggests a significant benefit for DMB with respect to time to first SRE. The rates of renal AEs were significantly lower in DMB pts while the overall rates of AEs, including hypocalcemia and ONJ, were consistent with the known DMB safety profile. The results of the landmark analysis and possible prolongation of PFS with DMB therapy is promising.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Multiple Myeloma, Bone disease


