
Contributions
Abstract: S498
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 16:30 - 16:45
Location: Room N104
Background
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, progressive, life-threatening disease caused by somatic phosphatidylinositol glycan class A (PIGA) gene mutation in bone marrow stem cells. The International PNH Registry (NCT01374360) is a prospective, multinational, observational study to record the natural history of PNH and collect data on long-term efficacy and safety of treatment with eculizumab (ecu), a humanized monoclonal antibody approved for treatment of PNH.
Aims
Evaluate the effect of ecu in patients with PNH with or without high disease activity (HDA).
Methods
Patients enrolled in the Registry as of December 5, 2016, were stratified by HDA and ecu treatment status into 4 groups: HDA/ecu-treated; HDA/never ecu-treated; no-HDA/ecu-treated; no-HDA/never ecu-treated. HDA is defined as lactate dehydrogenase (LDH) ratio ≥1.5x upper limit of normal within 6 months of baseline and history of any of the following: fatigue, hemoglobinuria, abdominal pain, dyspnea, anemia (hemoglobin <100 g/L), major adverse vascular event (MAVE; including thromboembolism [TE]), dysphagia, or erectile dysfunction. Patients were assessed at baseline (date of enrollment in never ecu-treated patients; date of initiation of ecu in ecu-treated patients) and at last follow-up. Outcomes include changes from baseline to last follow-up in LDH ratio, GPI-deficient granulocytes, red blood cell transfusions received, MAVE, and Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue score in patients with at least 6 months of follow-up.
Results
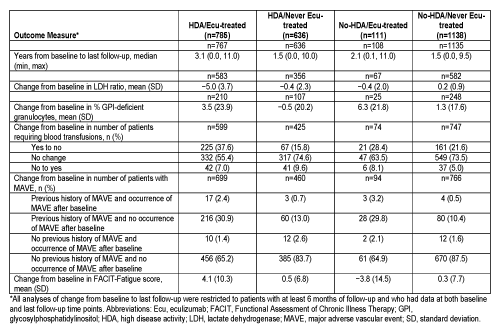
4717 patients were enrolled; of these, 2670 had non-missing data on ecu and HDA status, and were included in the current analysis (HDA/ecu-treated, n=785; HDA/never ecu-treated, n=636; no-HDA/ecu-treated, n=111; no-HDA/never ecu-treated, n=1138). Median (min, max) duration of follow-up after baseline was longer for the ecu-treated patients compared with the never ecu-treated patients for both the HDA and no-HDA groups (see Table). Results for changes from baseline to last follow-up in outcomes of interest are summarized in the Table. Data show that patients in the ecu-treated cohort had high burden of disease at baseline. Specifically, in the HDA population, a higher proportion of ecu-treated patients had a history of MAVE (33.3%) vs never ecu-treated patients (13.7%). A similar disparity at baseline was also observed in the no-HDA population (33.0% vs 11.0%, respectively). Following ecu treatment, the divergence in the proportion of patients with MAVE has substantially narrowed for the HDA patients (3.9% for ecu-treated vs 3.3% for never ecu-treated) despite longer follow-up for the treated patients. Similar findings were seen in no-HDA patients (5.3% vs 2.1% respectively). In patients with HDA status, treatment with ecu was associated with meaningful improvement in mean (standard deviation [SD]) reduction from baseline in LDH ratio (−5.0 [3.7] vs −0.4 [2.3]) and proportion of red blood cell transfusion-free patients (37.6% vs 15.8%). The FACIT-Fatigue data, while limited, showed the HDA/ecu-treated group experienced greater mean (SD) score improvement than the HDA/never ecu-treated group (4.1 [10.3] vs 0.5 [6.8] points).
Conclusion
Our analysis of real-world data from the International PNH Registry has demonstrated that treatment with eculizumab was associated with improved outcomes in patients with HDA. Our findings are consistent with the notion that patients with HDA, including those with a history of MAVE, should be treated with eculizumab.
Session topic: 12. Bone marrow failure syndromes incl. PNH - Clinical
Keyword(s): Thrombosis, Quality of Life, Paroxysmal nocturnal hemoglobinuria (PNH), Monoclonal antibody
Abstract: S498
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 16:30 - 16:45
Location: Room N104
Background
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, progressive, life-threatening disease caused by somatic phosphatidylinositol glycan class A (PIGA) gene mutation in bone marrow stem cells. The International PNH Registry (NCT01374360) is a prospective, multinational, observational study to record the natural history of PNH and collect data on long-term efficacy and safety of treatment with eculizumab (ecu), a humanized monoclonal antibody approved for treatment of PNH.
Aims
Evaluate the effect of ecu in patients with PNH with or without high disease activity (HDA).
Methods
Patients enrolled in the Registry as of December 5, 2016, were stratified by HDA and ecu treatment status into 4 groups: HDA/ecu-treated; HDA/never ecu-treated; no-HDA/ecu-treated; no-HDA/never ecu-treated. HDA is defined as lactate dehydrogenase (LDH) ratio ≥1.5x upper limit of normal within 6 months of baseline and history of any of the following: fatigue, hemoglobinuria, abdominal pain, dyspnea, anemia (hemoglobin <100 g/L), major adverse vascular event (MAVE; including thromboembolism [TE]), dysphagia, or erectile dysfunction. Patients were assessed at baseline (date of enrollment in never ecu-treated patients; date of initiation of ecu in ecu-treated patients) and at last follow-up. Outcomes include changes from baseline to last follow-up in LDH ratio, GPI-deficient granulocytes, red blood cell transfusions received, MAVE, and Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue score in patients with at least 6 months of follow-up.
Results
4717 patients were enrolled; of these, 2670 had non-missing data on ecu and HDA status, and were included in the current analysis (HDA/ecu-treated, n=785; HDA/never ecu-treated, n=636; no-HDA/ecu-treated, n=111; no-HDA/never ecu-treated, n=1138). Median (min, max) duration of follow-up after baseline was longer for the ecu-treated patients compared with the never ecu-treated patients for both the HDA and no-HDA groups (see Table). Results for changes from baseline to last follow-up in outcomes of interest are summarized in the Table. Data show that patients in the ecu-treated cohort had high burden of disease at baseline. Specifically, in the HDA population, a higher proportion of ecu-treated patients had a history of MAVE (33.3%) vs never ecu-treated patients (13.7%). A similar disparity at baseline was also observed in the no-HDA population (33.0% vs 11.0%, respectively). Following ecu treatment, the divergence in the proportion of patients with MAVE has substantially narrowed for the HDA patients (3.9% for ecu-treated vs 3.3% for never ecu-treated) despite longer follow-up for the treated patients. Similar findings were seen in no-HDA patients (5.3% vs 2.1% respectively). In patients with HDA status, treatment with ecu was associated with meaningful improvement in mean (standard deviation [SD]) reduction from baseline in LDH ratio (−5.0 [3.7] vs −0.4 [2.3]) and proportion of red blood cell transfusion-free patients (37.6% vs 15.8%). The FACIT-Fatigue data, while limited, showed the HDA/ecu-treated group experienced greater mean (SD) score improvement than the HDA/never ecu-treated group (4.1 [10.3] vs 0.5 [6.8] points).

Conclusion
Our analysis of real-world data from the International PNH Registry has demonstrated that treatment with eculizumab was associated with improved outcomes in patients with HDA. Our findings are consistent with the notion that patients with HDA, including those with a history of MAVE, should be treated with eculizumab.
Session topic: 12. Bone marrow failure syndromes incl. PNH - Clinical
Keyword(s): Thrombosis, Quality of Life, Paroxysmal nocturnal hemoglobinuria (PNH), Monoclonal antibody


