
Contributions
Abstract: S488
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 16:30 - 16:45
Location: Room N105
Background
Azacitidine (AZA) is first line therapy for patients (pts) with higher risk MDS and demonstrated efficacy in older pts with AML (Dombret et al, Blood 2015; Fenaux et al, J Clin Oncol 2010). Rigosertib (RIG) interferes with the RAS-binding domains of RAF kinases and inhibits the RAS-RAF-MEK and the PI3Ks pathways. In vitro, the combination of RIG with AZA synergistically inhibits growth and induces apoptosis of leukemic cells in a sequence-dependent manner (Skidan et al, AACR 2006). RIG’s effective inhibition of human hematopoietic tumor cell lines in vitro, favorable clinical adverse event (AE) profile, and its synergy with AZA suggests the potential value of combination treatment.
Aims
Phase I/II results are presented for pts with MDS, either hypomethylating (HMA) treatment naïve or progressing on or failing to respond to prior HMA, and those with AML (per WHO 2002 criteria) with relapsed or refractory disease
Methods
Oral RIG was administered twice daily on Day 1-21 of a 28-day cycle in escalating cohorts and then at the recommended Phase II dose (560 mg qAM and 280 mg qPM). AZA 75 mg/m2/d SC or IV was administered for 7 days starting on Day 8. A CBC was performed weekly, and a bone marrow aspirate/biopsy were performed at baseline, D29, and then every 8 weeks thereafter. Response was determined by IWG criteria for MDS (2006) and AML (2003). Stable disease in AML was defined as not meeting criteria for any other treatment response (CR, CRi, PR, disease progression, or treatment failure).
Results
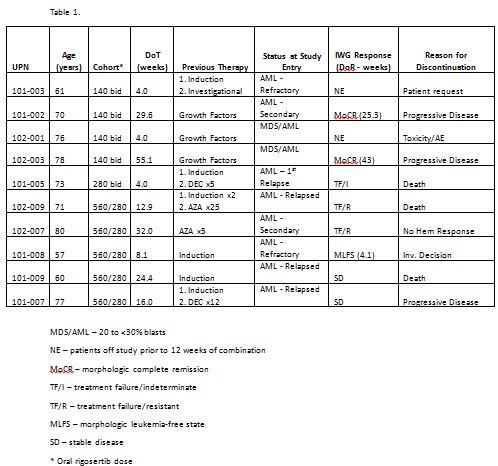
The combination of oral RIG and AZA was administered to 54 pts, of whom 40 had MDS; HMA-treatment-naïve (N=23) and previously HMA-failed pts (N=17). 17 MDS pts received prior HMA therapy: 12 AZA, 4 decitabine, and 1 both. Ten pts had AML, and 6 had CMML. 2 MDS patients with 20-<30% marrow blasts were also included in the AML analysis. Median age was 68 years; 67% of pts were male; and ECOG performance status was 0-1 in 95% of pts. Pts have received 1-37+ cycles of treatment (median, 3.5 cycles), with a median duration of treatment of 17 weeks (range 4 to 158+ weeks).
Conclusion
The combination of oral RIG and standard-dose AZA was well tolerated in repetitive cycles in pts with AML and MDS. Response was observed both in HMA-treatment-naïve pts (85%) and in pts failing HMA therapy (62%), suggesting the addition of RIG can overcome HMA clinical resistance by acting as a chromatin modifying agent. In AML, responses were seen in 37.5% of evaluable pts. Based on these results, continued study in AML is warranted. A Phase III study of the combination of oral RIG and AZA in pts with treatment naïve MDS is planned.
Session topic: 10. Myelodysplastic syndromes - Clinical
Keyword(s): Ras, MDS, Clinical Trial, AML
Abstract: S488
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 16:30 - 16:45
Location: Room N105
Background
Azacitidine (AZA) is first line therapy for patients (pts) with higher risk MDS and demonstrated efficacy in older pts with AML (Dombret et al, Blood 2015; Fenaux et al, J Clin Oncol 2010). Rigosertib (RIG) interferes with the RAS-binding domains of RAF kinases and inhibits the RAS-RAF-MEK and the PI3Ks pathways. In vitro, the combination of RIG with AZA synergistically inhibits growth and induces apoptosis of leukemic cells in a sequence-dependent manner (Skidan et al, AACR 2006). RIG’s effective inhibition of human hematopoietic tumor cell lines in vitro, favorable clinical adverse event (AE) profile, and its synergy with AZA suggests the potential value of combination treatment.
Aims
Phase I/II results are presented for pts with MDS, either hypomethylating (HMA) treatment naïve or progressing on or failing to respond to prior HMA, and those with AML (per WHO 2002 criteria) with relapsed or refractory disease
Methods
Oral RIG was administered twice daily on Day 1-21 of a 28-day cycle in escalating cohorts and then at the recommended Phase II dose (560 mg qAM and 280 mg qPM). AZA 75 mg/m2/d SC or IV was administered for 7 days starting on Day 8. A CBC was performed weekly, and a bone marrow aspirate/biopsy were performed at baseline, D29, and then every 8 weeks thereafter. Response was determined by IWG criteria for MDS (2006) and AML (2003). Stable disease in AML was defined as not meeting criteria for any other treatment response (CR, CRi, PR, disease progression, or treatment failure).
Results
The combination of oral RIG and AZA was administered to 54 pts, of whom 40 had MDS; HMA-treatment-naïve (N=23) and previously HMA-failed pts (N=17). 17 MDS pts received prior HMA therapy: 12 AZA, 4 decitabine, and 1 both. Ten pts had AML, and 6 had CMML. 2 MDS patients with 20-<30% marrow blasts were also included in the AML analysis. Median age was 68 years; 67% of pts were male; and ECOG performance status was 0-1 in 95% of pts. Pts have received 1-37+ cycles of treatment (median, 3.5 cycles), with a median duration of treatment of 17 weeks (range 4 to 158+ weeks).

Conclusion
The combination of oral RIG and standard-dose AZA was well tolerated in repetitive cycles in pts with AML and MDS. Response was observed both in HMA-treatment-naïve pts (85%) and in pts failing HMA therapy (62%), suggesting the addition of RIG can overcome HMA clinical resistance by acting as a chromatin modifying agent. In AML, responses were seen in 37.5% of evaluable pts. Based on these results, continued study in AML is warranted. A Phase III study of the combination of oral RIG and AZA in pts with treatment naïve MDS is planned.
Session topic: 10. Myelodysplastic syndromes - Clinical
Keyword(s): Ras, MDS, Clinical Trial, AML


