UPDATED SAFETY AND EFFICACY RESULTS OF PHASE 1/2 STUDY OF VENETOCLAX PLUS LOW-DOSE CYTARABINE IN TREATMENT-NAÏVE ACUTE MYELOID LEUKEMIA PATIENTS AGED ≥65 YEARS AND UNFIT FOR STANDARD INDUCTION THERAPY
(Abstract release date: 05/18/17)
EHA Library. H. Wei A. 06/24/17; 181760; S473

Andrew H. Wei
Contributions
Contributions
Abstract
Abstract: S473
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 16:30 - 16:45
Location: Hall D
Background
Incidence of acute myeloid leukemia (AML) increases with age, and patients (pts) ≥65 years have a poor prognosis, with 5-year survival rates of <10%. Treatment with low-dose cytarabine (LDAC) in this population results in modest complete remission (CR)/CR with incomplete blood count recovery (CRi) rates of 11–19% and median survival of 5-6 months. Venetoclax (VEN), an orally bioavailable, potent, selective BCL-2 inhibitor, has shown single-agent activity in heavily pretreated pts with relapsed/refractory AML (Konopleva et al 2016). In combination with LDAC, the recommended phase 2 dose (RP2D) of VEN is 600 mg QD (Lin et al, ASCO 2016); preliminary data show the combination is tolerable and exhibits significant and durable activity in older AML pts ineligible to receive intensive induction chemotherapy (Wei et al, ASH 2016). Updated safety and efficacy data from the RP2D 600-mg dose cohorts of this study (NCT02287233) are presented.
Aims
Evaluate the safety and efficacy of VEN+LDAC in older pts with untreated AML.
Methods
In this open-label phase 1/2 study, pts ≥65 years with untreated AML, ineligible for standard induction chemotherapy, with an ECOG performance status of 0–2 received oral VEN QD on days (d) 1–28 and subcutaneous LDAC 20 mg/m2 QD on d 1–10 of each 28-d cycle. VEN target dose evaluation followed a 3+3 design, ranging from 600–800 mg; 18 pts were enrolled and the RP2D was established as 600 mg. Safety and efficacy of VEN at RP2D were evaluated in the expansion phase. All pts were hospitalized and received prophylaxis before a dose ramp-up of VEN during cycle 1 to mitigate the risk of tumor lysis syndrome (TLS). Adverse events (AEs) were graded by NCI CTCAE V4.0. Pts enrolled as of May 2016 are included in this analysis; data cutoff was August 2016. All pts provided informed consent.
Results
In total, 61 pts, including 8 from phase 1, were treated at the RP2D of 600 mg (median age 74 years; ECOG 1–2 70%; adverse karyotypes 31%; secondary AML 44%; prior hypomethylating agent [HMA] 28%). AEs (all grade; ≥30% pts; excluding cytopenias) were nausea (72%), hypokalemia (46%), diarrhea (44%), fatigue (43%), and decreased appetite (41%). Grade 3/4 AEs (≥10% pts) were febrile neutropenia (34%), hypokalemia (15%), hypophosphatemia (13%), and hypertension (10%). No pts had clinical TLS; 1 pt had laboratory TLS, which was managed. The 30-d and 60-d mortality rates were 3% and 15%, respectively. The CR/CRi rate was 54% (33/61; 21% CR and 33% CRi). The overall response rate (ORR; CR+CRi+partial remission) was 61% (37/61). VEN+LDAC was shown to be active across a wide range of cytogenetic mutations and pt profiles (ORR: 70% in pts ≥75 years; 52% in secondary AML; 47% in pts with adverse karyotypes; 53% in pts with prior HMA). Among response-evaluable pts, those achieving an objective response have longer survival than pts who do not achieve an objective response (Figure).
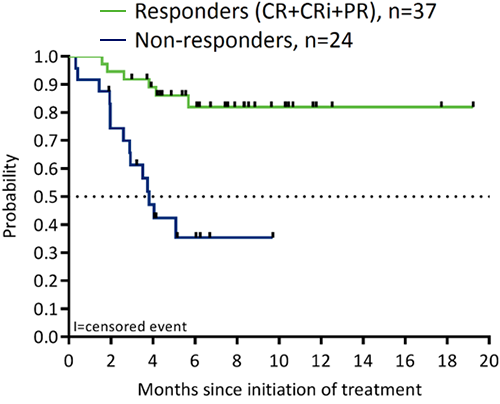
Conclusion
VEN (RP2D 600 mg) and LDAC exhibited an acceptable safety profile and durable efficacy in pts aged ≥65 years with untreated AML who are ineligible for or unable to receive intensive induction chemotherapy. ORR highly correlated with overall survival, with better survival observed in responders compared with nonresponders. A planned phase 3 randomized trial has commenced.
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): Elderly, BCL2, AML, Phase I/II
Abstract: S473
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 16:30 - 16:45
Location: Hall D
Background
Incidence of acute myeloid leukemia (AML) increases with age, and patients (pts) ≥65 years have a poor prognosis, with 5-year survival rates of <10%. Treatment with low-dose cytarabine (LDAC) in this population results in modest complete remission (CR)/CR with incomplete blood count recovery (CRi) rates of 11–19% and median survival of 5-6 months. Venetoclax (VEN), an orally bioavailable, potent, selective BCL-2 inhibitor, has shown single-agent activity in heavily pretreated pts with relapsed/refractory AML (Konopleva et al 2016). In combination with LDAC, the recommended phase 2 dose (RP2D) of VEN is 600 mg QD (Lin et al, ASCO 2016); preliminary data show the combination is tolerable and exhibits significant and durable activity in older AML pts ineligible to receive intensive induction chemotherapy (Wei et al, ASH 2016). Updated safety and efficacy data from the RP2D 600-mg dose cohorts of this study (NCT02287233) are presented.
Aims
Evaluate the safety and efficacy of VEN+LDAC in older pts with untreated AML.
Methods
In this open-label phase 1/2 study, pts ≥65 years with untreated AML, ineligible for standard induction chemotherapy, with an ECOG performance status of 0–2 received oral VEN QD on days (d) 1–28 and subcutaneous LDAC 20 mg/m2 QD on d 1–10 of each 28-d cycle. VEN target dose evaluation followed a 3+3 design, ranging from 600–800 mg; 18 pts were enrolled and the RP2D was established as 600 mg. Safety and efficacy of VEN at RP2D were evaluated in the expansion phase. All pts were hospitalized and received prophylaxis before a dose ramp-up of VEN during cycle 1 to mitigate the risk of tumor lysis syndrome (TLS). Adverse events (AEs) were graded by NCI CTCAE V4.0. Pts enrolled as of May 2016 are included in this analysis; data cutoff was August 2016. All pts provided informed consent.
Results
In total, 61 pts, including 8 from phase 1, were treated at the RP2D of 600 mg (median age 74 years; ECOG 1–2 70%; adverse karyotypes 31%; secondary AML 44%; prior hypomethylating agent [HMA] 28%). AEs (all grade; ≥30% pts; excluding cytopenias) were nausea (72%), hypokalemia (46%), diarrhea (44%), fatigue (43%), and decreased appetite (41%). Grade 3/4 AEs (≥10% pts) were febrile neutropenia (34%), hypokalemia (15%), hypophosphatemia (13%), and hypertension (10%). No pts had clinical TLS; 1 pt had laboratory TLS, which was managed. The 30-d and 60-d mortality rates were 3% and 15%, respectively. The CR/CRi rate was 54% (33/61; 21% CR and 33% CRi). The overall response rate (ORR; CR+CRi+partial remission) was 61% (37/61). VEN+LDAC was shown to be active across a wide range of cytogenetic mutations and pt profiles (ORR: 70% in pts ≥75 years; 52% in secondary AML; 47% in pts with adverse karyotypes; 53% in pts with prior HMA). Among response-evaluable pts, those achieving an objective response have longer survival than pts who do not achieve an objective response (Figure).

Conclusion
VEN (RP2D 600 mg) and LDAC exhibited an acceptable safety profile and durable efficacy in pts aged ≥65 years with untreated AML who are ineligible for or unable to receive intensive induction chemotherapy. ORR highly correlated with overall survival, with better survival observed in responders compared with nonresponders. A planned phase 3 randomized trial has commenced.
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): Elderly, BCL2, AML, Phase I/II
{{ help_message }}
{{filter}}


