SAFETY AND EFFICACY OF VENETOCLAX (VEN) IN COMBINATION WITH DECITABINE OR AZACITIDINE IN TREATMENT-NAIVE, ELDERLY PATIENTS (≥65 YEARS) WITH ACUTE MYELOID LEUKEMIA (AML)
(Abstract release date: 05/18/17)
EHA Library. Pratz K. 06/24/17; 181759; S472

Dr. Keith Pratz
Contributions
Contributions
Abstract
Abstract: S472
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 16:15 - 16:30
Location: Hall D
Background
Newly diagnosed patients (pts) with AML aged ≥65 years and ineligible for standard induction therapy have limited treatment options, and low overall survival. VEN is an orally bioavailable, selective BCL-2 inhibitor that has displayed single-agent activity in pts with relapsed/refractory AML. VEN at escalating doses combined with hypomethylating agents (HMAs) has demonstrated antileukemic activity, with an overall response rate (ORR) including complete remission [CR], and CR with incomplete marrow recovery of 60%. Combining VEN with HMAs, such as decitabine (DEC) or azacitidine (AZA), may provide a novel low-intensity approach for treating AML. Preliminary results from the expansion stage of a phase 1b trial comparing 2 doses of VEN plus either DEC or AZA (NCT02203773) are reported.
Aims
To evaluate the safety and efficacy of VEN at 400-mg vs 800-mg doses plus DEC or AZA.
Methods
This open-label, nonrandomized, two-stage phase 1b study evaluated the safety and efficacy of VEN plus DEC or AZA in treatment-naive pts ≥65 years with AML. Eligibility included: ECOG PS ≤2; ineligible for standard induction therapy; intermediate- or poor-risk karyotype. Pts received DEC (Arm D, 20 mg/m2/day [d]; intravenous [IV]) on d 1–5, or AZA (Arm E, 75 mg/m2/d; subcutaneous or IV) on d 1–7 of each 28-d cycle (C) in combination with once-daily oral VEN. The dose-expansion stage consisted of 2 VEN dose cohorts (continuous 400-mg and interrupted 800-mg dosing) in each arm (D1, D2, E1, and E2, respectively) to determine optimal dose. Tumor lysis syndrome (TLS) prophylaxis was administered in C1 to all pts during VEN dose ramp-up until final dose was reached. All pts provided informed consent.
Results
As of 13/09/16, 100 pts were enrolled in the expansion stage: 25 pts in each arm. Overall, 61% pts were male; 59% had ECOG PS 1 and 15% ECOG PS 2; mean age was 73.9 (range 65–86); 53% had adverse karyotype; and 22% had secondary AML. Median time on study was 6 (4–9), 6 (0.2–9), 5 (0.5–9), and 4 (1–8) mo for arms D1, D2, E1, and E2, respectively.
The incidence of adverse events (AEs) was generally comparable between the 4 arms. Overall, the most common treatment-emergent AEs (TEAEs; in ≥30% of pts) were nausea (59%), diarrhea (42%), febrile neutropenia (FN; 41%), constipation (39%), fatigue, and decreased white blood cell count (31% each). The most frequent grade 3/4 TEAE and serious AE was FN (41% and 29%, respectively). No TLS was observed. Overall, 29 pts discontinued the study for ≥1 reason, including progressive disease (PD) per protocol (n=10), “other” (n=10; 9/10 proceeded to stem cell transplantation) and AEs not related to progression (n=10). A total of 16 deaths occurred; 12 pts died within 30 d of initiating VEN and HMA due to AEs (n=12) and PD (n=1).
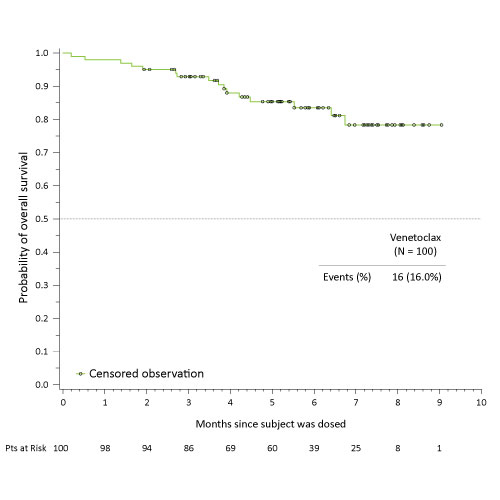
The ORR was 68%, with rates of 76% (19/25), 71% (17/24), 68% (17/25), and 60% (15/25) observed in arms D1, D2, E1, and E2, respectively. The Kaplan-Meier survival curve for all pts with a median follow-up time of 5.4 mo is shown.
Conclusion
Overall, the safety profile was favorable when combining VEN at either dose with DEC or AZA in treatment-naive elderly AML pts. Promising activity with high ORRs was observed at the lower 400-mg VEN dose in both HMA arms. A Phase 3 study of VEN plus AZA is planned.
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): Phase I, Elderly, BCL2, AML
Abstract: S472
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 16:15 - 16:30
Location: Hall D
Background
Newly diagnosed patients (pts) with AML aged ≥65 years and ineligible for standard induction therapy have limited treatment options, and low overall survival. VEN is an orally bioavailable, selective BCL-2 inhibitor that has displayed single-agent activity in pts with relapsed/refractory AML. VEN at escalating doses combined with hypomethylating agents (HMAs) has demonstrated antileukemic activity, with an overall response rate (ORR) including complete remission [CR], and CR with incomplete marrow recovery of 60%. Combining VEN with HMAs, such as decitabine (DEC) or azacitidine (AZA), may provide a novel low-intensity approach for treating AML. Preliminary results from the expansion stage of a phase 1b trial comparing 2 doses of VEN plus either DEC or AZA (NCT02203773) are reported.
Aims
To evaluate the safety and efficacy of VEN at 400-mg vs 800-mg doses plus DEC or AZA.
Methods
This open-label, nonrandomized, two-stage phase 1b study evaluated the safety and efficacy of VEN plus DEC or AZA in treatment-naive pts ≥65 years with AML. Eligibility included: ECOG PS ≤2; ineligible for standard induction therapy; intermediate- or poor-risk karyotype. Pts received DEC (Arm D, 20 mg/m2/day [d]; intravenous [IV]) on d 1–5, or AZA (Arm E, 75 mg/m2/d; subcutaneous or IV) on d 1–7 of each 28-d cycle (C) in combination with once-daily oral VEN. The dose-expansion stage consisted of 2 VEN dose cohorts (continuous 400-mg and interrupted 800-mg dosing) in each arm (D1, D2, E1, and E2, respectively) to determine optimal dose. Tumor lysis syndrome (TLS) prophylaxis was administered in C1 to all pts during VEN dose ramp-up until final dose was reached. All pts provided informed consent.
Results
As of 13/09/16, 100 pts were enrolled in the expansion stage: 25 pts in each arm. Overall, 61% pts were male; 59% had ECOG PS 1 and 15% ECOG PS 2; mean age was 73.9 (range 65–86); 53% had adverse karyotype; and 22% had secondary AML. Median time on study was 6 (4–9), 6 (0.2–9), 5 (0.5–9), and 4 (1–8) mo for arms D1, D2, E1, and E2, respectively.
The incidence of adverse events (AEs) was generally comparable between the 4 arms. Overall, the most common treatment-emergent AEs (TEAEs; in ≥30% of pts) were nausea (59%), diarrhea (42%), febrile neutropenia (FN; 41%), constipation (39%), fatigue, and decreased white blood cell count (31% each). The most frequent grade 3/4 TEAE and serious AE was FN (41% and 29%, respectively). No TLS was observed. Overall, 29 pts discontinued the study for ≥1 reason, including progressive disease (PD) per protocol (n=10), “other” (n=10; 9/10 proceeded to stem cell transplantation) and AEs not related to progression (n=10). A total of 16 deaths occurred; 12 pts died within 30 d of initiating VEN and HMA due to AEs (n=12) and PD (n=1).
The ORR was 68%, with rates of 76% (19/25), 71% (17/24), 68% (17/25), and 60% (15/25) observed in arms D1, D2, E1, and E2, respectively. The Kaplan-Meier survival curve for all pts with a median follow-up time of 5.4 mo is shown.

Conclusion
Overall, the safety profile was favorable when combining VEN at either dose with DEC or AZA in treatment-naive elderly AML pts. Promising activity with high ORRs was observed at the lower 400-mg VEN dose in both HMA arms. A Phase 3 study of VEN plus AZA is planned.
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): Phase I, Elderly, BCL2, AML
{{ help_message }}
{{filter}}


