
Contributions
Abstract: S471
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 16:00 - 16:15
Location: Hall D
Background
Recurrent mutations in isocitrate dehydrogenase 2 (mIDH2) occur in ~12% of AML patients (pts). mIDH2 proteins synthesize an oncometabolite, 2-hydroxyglutarate (2HG), causing DNA and histone hypermethylation and blocked myeloid differentiation. Enasidenib (AG-221) is an oral, selective, small-molecule inhibitor of mIDH2 proteins. Differentiation of myeloblasts, not cytotoxicity, appears to drive the clinical efficacy of enasidenib. In preclinical studies, bone marrow blasts from pts with mIDH2 AML exposed to enasidenib ex vivo were shown to produce mature, fully functioning neutrophils with conserved mIDH2 allele frequency, indicating differentiation of mature cells from the mIDH2 blasts (Yen et al, Cancer Discov, 2017). Additionally, no apoptosis was observed in mIDH2-R140 erythroleukemia (TF-1) cells treated with enasidenib for 7 days in vitro.
Aims
Evaluate the maximum tolerated dose (MTD), pharmacokinetic (PK) and pharmacodynamic (PD) profiles, safety, and clinical activity of enasidenib in pts with mIDH2 advanced myeloid malignancies.
Methods
This phase 1/2 study included pts aged ≥18 years (yrs) with mIDH2 WHO-defined AML, or with mIDH2 MDS with refractory anemia with excess blasts, and ECOG PS scores ≤2. Pts were relapsed or refractory (R/R) to prior anti-cancer therapy, or had untreated AML if aged ≥60 years and not eligible for standard-of-care treatment (Tx). Safety for all pts and clinical efficacy in the largest pt subgroup, those with R/R AML, from the phase 1 dose-escalation and expansion phases are reported.
Results
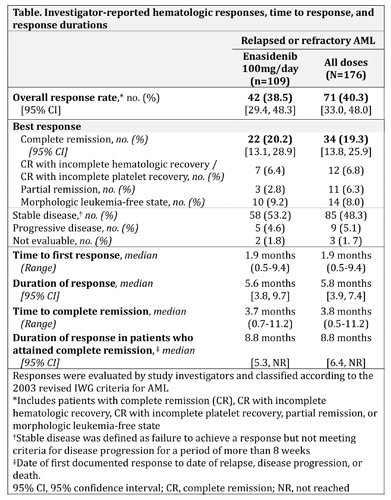
In all, 239 pts received enasidenib. Median age was 70 yrs. In the dose-escalation phase (n=113), pts received daily enasidenib doses of 50-650mg. The MTD was not reached. Median 2HG reductions from baseline at cycle 2 day 1 were 92%, 90%, and 93% for pts receiving <100mg, 100mg, and >100mg/day, respectively. Enasidenib 100mg QD was chosen for the expansion phase (n=126) based on PK/PD profiles and demonstrated efficacy. Median number of enasidenib cycles was 5 (range 1–25). Grade 3-4 investigator-reported Tx-related adverse events included indirect hyperbilirubinemia (12%) and IDH-inhibitor-associated differentiation syndrome (IDH-DS; ie, retinoic acid syndrome) (7%). Of 176 R/R AML pts, 94 (53%) had received ≥2 prior AML-directed Tx. Overall response rate (ORR; complete remission [CR] + CR with incomplete hematologic recovery + morphologic leukemia-free state + partial remission) in R/R AML pts was 40.3%, including 34 pts (19.3%) who attained CR (Table). Median time to 1st response was 1.9 months (mos); 87.3% of responding pts attained a 1st response by cycle 5. Median response duration was 5.8 mos. Of pts who achieved CR, 7 pts (21%) did so by cycle 3, 23 (68%) by cycle 5, and 28 (82%) by cycle 7. Median duration of CR was 8.8 mos. ORR with enasidenib 100mg/day was 38.5% (Table). Seventeen pts (11%) proceeded to stem cell transplant. Response was associated with cellular differentiation, typically with no evidence of aplasia. Median overall survival (OS) of R/R AML pts was 9.3 mos. For pts who attained CR, OS was 19.7 mos. Pts who had received ≥2 prior AML Tx had a median OS of 8.0 mos.
Conclusion
Enasidenib was well tolerated, induced CRs in R/R AML pts, and was associated with OS of >9 mos in pts who had failed prior AML Tx. A randomized phase 3 study of enasidenib vs conventional care in older pts with late-stage R/R AML is ongoing (NCT02577406).
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): Phase I/II, Mutation, Clinical Trial, Acute Myeloid Leukemia
Abstract: S471
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 16:00 - 16:15
Location: Hall D
Background
Recurrent mutations in isocitrate dehydrogenase 2 (mIDH2) occur in ~12% of AML patients (pts). mIDH2 proteins synthesize an oncometabolite, 2-hydroxyglutarate (2HG), causing DNA and histone hypermethylation and blocked myeloid differentiation. Enasidenib (AG-221) is an oral, selective, small-molecule inhibitor of mIDH2 proteins. Differentiation of myeloblasts, not cytotoxicity, appears to drive the clinical efficacy of enasidenib. In preclinical studies, bone marrow blasts from pts with mIDH2 AML exposed to enasidenib ex vivo were shown to produce mature, fully functioning neutrophils with conserved mIDH2 allele frequency, indicating differentiation of mature cells from the mIDH2 blasts (Yen et al, Cancer Discov, 2017). Additionally, no apoptosis was observed in mIDH2-R140 erythroleukemia (TF-1) cells treated with enasidenib for 7 days in vitro.
Aims
Evaluate the maximum tolerated dose (MTD), pharmacokinetic (PK) and pharmacodynamic (PD) profiles, safety, and clinical activity of enasidenib in pts with mIDH2 advanced myeloid malignancies.
Methods
This phase 1/2 study included pts aged ≥18 years (yrs) with mIDH2 WHO-defined AML, or with mIDH2 MDS with refractory anemia with excess blasts, and ECOG PS scores ≤2. Pts were relapsed or refractory (R/R) to prior anti-cancer therapy, or had untreated AML if aged ≥60 years and not eligible for standard-of-care treatment (Tx). Safety for all pts and clinical efficacy in the largest pt subgroup, those with R/R AML, from the phase 1 dose-escalation and expansion phases are reported.
Results
In all, 239 pts received enasidenib. Median age was 70 yrs. In the dose-escalation phase (n=113), pts received daily enasidenib doses of 50-650mg. The MTD was not reached. Median 2HG reductions from baseline at cycle 2 day 1 were 92%, 90%, and 93% for pts receiving <100mg, 100mg, and >100mg/day, respectively. Enasidenib 100mg QD was chosen for the expansion phase (n=126) based on PK/PD profiles and demonstrated efficacy. Median number of enasidenib cycles was 5 (range 1–25). Grade 3-4 investigator-reported Tx-related adverse events included indirect hyperbilirubinemia (12%) and IDH-inhibitor-associated differentiation syndrome (IDH-DS; ie, retinoic acid syndrome) (7%). Of 176 R/R AML pts, 94 (53%) had received ≥2 prior AML-directed Tx. Overall response rate (ORR; complete remission [CR] + CR with incomplete hematologic recovery + morphologic leukemia-free state + partial remission) in R/R AML pts was 40.3%, including 34 pts (19.3%) who attained CR (Table). Median time to 1st response was 1.9 months (mos); 87.3% of responding pts attained a 1st response by cycle 5. Median response duration was 5.8 mos. Of pts who achieved CR, 7 pts (21%) did so by cycle 3, 23 (68%) by cycle 5, and 28 (82%) by cycle 7. Median duration of CR was 8.8 mos. ORR with enasidenib 100mg/day was 38.5% (Table). Seventeen pts (11%) proceeded to stem cell transplant. Response was associated with cellular differentiation, typically with no evidence of aplasia. Median overall survival (OS) of R/R AML pts was 9.3 mos. For pts who attained CR, OS was 19.7 mos. Pts who had received ≥2 prior AML Tx had a median OS of 8.0 mos.

Conclusion
Enasidenib was well tolerated, induced CRs in R/R AML pts, and was associated with OS of >9 mos in pts who had failed prior AML Tx. A randomized phase 3 study of enasidenib vs conventional care in older pts with late-stage R/R AML is ongoing (NCT02577406).
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): Phase I/II, Mutation, Clinical Trial, Acute Myeloid Leukemia


