Abstract: S468
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 16:30 - 16:45
Location: Hall C
Background
Transplant ineligible patients (pts) with relapsed/refractory (R/R) FL or DLBCL have poor outcomes. Polatuzumab vedotin (pola) is an antibody drug conjugate that targets delivery of the microtubule inhibitor MMAE to cells expressing CD79b. Pola + rituximab (R) previously showed promising responses in R/R FL and DLBCL. Adding bendamustine (B) to pola-R and substituting obinutuzumab (G) for R could improve outcomes. We report updated results from the Phase 1b/2 (P1b/2) study evaluating pola + BR or BG in R/R FL and DLBCL and the expansion cohorts evaluating pola + BG in R/R FL and DLBCL (ClinicalTrials.gov NCT02257567).
Aims
The primary aim is to assess safety and tolerability of pola + BR/BG in R/R FL and DLBCL. Secondary aims include assessing safety and efficacy of pola + BG in an expansion cohort.
Methods
All pts provided informed consent to participate in the study and were treated with pola (1.8 mg/kg) + B (90 mg/m2) and R (375 mg/m2) or G (1000 mg) every 28 days (FL) or 21 days (DLBCL) for 6 cycles. Responses were assessed by modified Lugano 2014 criteria after 3 cycles, end of treatment (tx), and every 6 months for 2 years during follow-up (fu).
Results
As of 14 Nov 2016, 65 pts were enrolled: 24 pts (12 FL, 12 DLBCL) in P1b and 41 pts (20 FL and 21 DLBCL) in P2. In safety evaluable pts, FL pts (N=32) were median age of 63 yr (37-86), 82% ECOG 0-1 and 6% ECOG 2, 44% FLIPI1 3-5, 78% Stage III/IV, 2 (1-7) median lines of prior tx, 38% refractory to last tx, 13% prior transplant (BMT). DLBCL pts (N=32) were median age 66 (30-86), 88% ECOG 0-1 and 13% ECOG 2, 59% IPI 3-5, 75% Stage III/IV, 2 (1-7) median lines of prior tx, 82% refractory to last tx, 3% prior BMT.
Among 64 pts who received ≥1 dose, the adverse events (AEs) that occurred in >20% of pts were: fatigue (67%), nausea (54%), diarrhea (54%), vomiting (42%), pyrexia (39%) and constipation (39%). As expected, grade (Gr) 3/4 cytopenias were common: neutropenia (34% FL, 28% DLBCL), thrombcytopenia (16% FL, 13% DLBCL), anemia (6% FL, 9% DLBCL). Tx emergent neuropathy occurred in 19/64 (30%) of pts, with 1 Gr 3 event, and led to pola discontinuation in 1 pt, dose reduction in 2 pts, and interruption in 1 pt.
In FL, 75% (24/32) had Gr 3/4 AEs and 41% (13/32) had serious AEs (SAEs). The only SAE occurring in ≥10% was infection (22%). The most common Gr 3/4 non-heme AEs were infection (16%) and hypokalemia (9%). AEs led to study tx discontinuation in 6 pts. B was stopped in 2 pts due to Gr 3 thromboctyopenia. Of 4 deaths: 2 were PD and 2 were Gr 5 AEs, 1 tx related (PML), 1 tx unrelated. In DLBCL, 88% (28/32) had Gr 3/4 AEs and 63% (20/32) had SAEs. Most common Gr 3/4 non-heme AEs were febrile neutropenia (13%), fatigue (13%), and diarrhea (13%). SAEs occurring in ≥10% of pts were infection (33%) and pyrexia (22%). AEs led to study tx interruption in 19 pts and discontinuation in 8 pts. There were 13 deaths: 9 PD, 4 AE (all unrelated to tx).
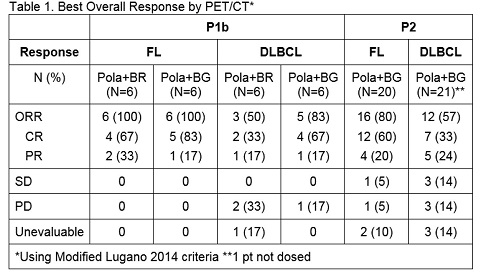
Responses by modified Lugano 2014 criteria are shown in Table1.
Median duration of response (DoR) for FL P1b pts was 16 months (mo)(median fu 14.5 mo). Median DoR for FL P2 (median fu 6.5 mo) and DLBCL P1b/2 (median fu 13.7 mo P1b, 6.4 mo P2) have not been reached.
Conclusion
Updated evaluation of pola + BR shows promising durable responses and an acceptable safety profile in heavily pre-treated R/R FL and DLBCL pts. Safety and efficacy data will be updated at the time of presentation.
Session topic: 20. Aggressive Non-Hodgkin lymphoma - Clinical





