
Contributions
Abstract: S467
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 16:15 - 16:30
Location: Hall C
Background
CC-122 is a cereblon modulating agent that degrades Aiolos and Ikaros, resulting in potent anti-lymphoma and immunomodulatory effects on T- and NK-cell function. Phase I clinical data revealed promising activity of CC-122 against follicular lymphoma (FL) and diffuse large B-cell lymphoma (DLBCL). Preclinical combination of CC-122 with obinutuzumab has shown synergism in FL and additive effects in DLBCL vs either single agent (Chiu. ASH 2015), supporting further study of this combination’s therapeutic potential.
Aims
The current phase Ib study (EUDRACT 2014-003333-26; NCT02417285) evaluates the safety and efficacy of CC-122 plus obinutuzumab in patients with relapsed or refractory (R/R) B-cell non-Hodgkin lymphoma (NHL).
Methods
Patients at study entry must have R/R CD20+ B-cell NHL after ≥1 prior regimens for FL/marginal zone lymphoma (MZL) and ≥2 regimens and/or ASCT for DLBCL. CC-122 was given orally (5 of 7 d) for 28-d cycles in escalating doses plus a fixed dose of intravenous obinutuzumab 1000 mg on d2, 8, 15 of cycle 1 (c1) and d1 of c2-8, upon informed consent. CC-122 was continued until progressive disease (PD) or unacceptable toxicity. CC-122 active ingredient in capsule formulation (AIC) 1, 2, 3, and 4 mg and CC-122 formulated capsules (F6) 3 and 4 mg were evaluated in separate cohorts. Primary endpoints included safety and tolerability, non-tolerated dose (NTD), and maximum tolerated dose (MTD). Response was assessed using the international Cheson 2007 criteria every 2 cycles to c6, every 3 cycles to c12, and every 6 cycles thereafter.
Results
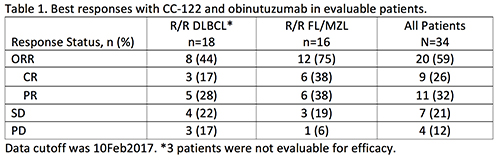
As of January 12, 2017, 34 R/R B-cell NHL patients with DLBCL (n=18), FL (n=15), or MZL (n=1) were enrolled. At study entry, median age was 60 y (26-81), most patients were male (68%), and Ann Arbor was extended stage III/IV in 76% of patients. Of the 18 DLBCL patients, 8 had transformed FL. Of the 16 FL/MZL patients, 44% relapsed in <12 months after first-line treatment. The median number of prior regimens was 4 (range, 1-11), and 13 (38%) patients had received prior SCT. One patient experienced a dose-limiting toxicity (DLT) of grade 4 neutropenia (CC-122 dose level of AIC 3 mg); no dose was yet an NTD. Median CC-122 duration was 22 wks (range, 3-71) equivalent to 6 cycles (range, 1-18). CC-122 dose reduction or temporary interruption occurred in 10 (29%) or 26 (76%) of patients, respectively, primarily due to adverse events (AEs). Most patients (56%) had <1 wk of interruption due to AEs. The most common (≥10%) grade 3/4 treatment-emergent AEs (TEAEs) were neutropenia (50%) and thrombocytopenia (21%). Fifteen patients (44%) had ≥1 serious TEAE, including 2 each of febrile neutropenia (related to CC-122), cytokine release syndrome (related to obinutuzumab), and pneumonia. Three deaths occurred during the study (2 PD; 1 AE-related). Overall response rate (ORR) was 59%, including 26% CR and 32% PR (Table 1). Median time to best response was 57 d, and median duration of response was not yet reached. In evaluable patients, 6-mo progression-free survival (PFS) was 63%.
Conclusion
The combination of CC-122 and obinutuzumab was well tolerated and demonstrates promising response rates and durable remissions in R/R patients with B-cell NHL. CC-122 doses of ≥3 mg and obinutuzumab have shown best response rates to date. The study is ongoing to establish the phase II recommended dose.
Session topic: 20. Aggressive Non-Hodgkin lymphoma - Clinical
Keyword(s): Relapse, Obinutuzumab, Non-Hodgkin's lymphoma, B cell lymphoma
Abstract: S467
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 16:15 - 16:30
Location: Hall C
Background
CC-122 is a cereblon modulating agent that degrades Aiolos and Ikaros, resulting in potent anti-lymphoma and immunomodulatory effects on T- and NK-cell function. Phase I clinical data revealed promising activity of CC-122 against follicular lymphoma (FL) and diffuse large B-cell lymphoma (DLBCL). Preclinical combination of CC-122 with obinutuzumab has shown synergism in FL and additive effects in DLBCL vs either single agent (Chiu. ASH 2015), supporting further study of this combination’s therapeutic potential.
Aims
The current phase Ib study (EUDRACT 2014-003333-26; NCT02417285) evaluates the safety and efficacy of CC-122 plus obinutuzumab in patients with relapsed or refractory (R/R) B-cell non-Hodgkin lymphoma (NHL).
Methods
Patients at study entry must have R/R CD20+ B-cell NHL after ≥1 prior regimens for FL/marginal zone lymphoma (MZL) and ≥2 regimens and/or ASCT for DLBCL. CC-122 was given orally (5 of 7 d) for 28-d cycles in escalating doses plus a fixed dose of intravenous obinutuzumab 1000 mg on d2, 8, 15 of cycle 1 (c1) and d1 of c2-8, upon informed consent. CC-122 was continued until progressive disease (PD) or unacceptable toxicity. CC-122 active ingredient in capsule formulation (AIC) 1, 2, 3, and 4 mg and CC-122 formulated capsules (F6) 3 and 4 mg were evaluated in separate cohorts. Primary endpoints included safety and tolerability, non-tolerated dose (NTD), and maximum tolerated dose (MTD). Response was assessed using the international Cheson 2007 criteria every 2 cycles to c6, every 3 cycles to c12, and every 6 cycles thereafter.
Results
As of January 12, 2017, 34 R/R B-cell NHL patients with DLBCL (n=18), FL (n=15), or MZL (n=1) were enrolled. At study entry, median age was 60 y (26-81), most patients were male (68%), and Ann Arbor was extended stage III/IV in 76% of patients. Of the 18 DLBCL patients, 8 had transformed FL. Of the 16 FL/MZL patients, 44% relapsed in <12 months after first-line treatment. The median number of prior regimens was 4 (range, 1-11), and 13 (38%) patients had received prior SCT. One patient experienced a dose-limiting toxicity (DLT) of grade 4 neutropenia (CC-122 dose level of AIC 3 mg); no dose was yet an NTD. Median CC-122 duration was 22 wks (range, 3-71) equivalent to 6 cycles (range, 1-18). CC-122 dose reduction or temporary interruption occurred in 10 (29%) or 26 (76%) of patients, respectively, primarily due to adverse events (AEs). Most patients (56%) had <1 wk of interruption due to AEs. The most common (≥10%) grade 3/4 treatment-emergent AEs (TEAEs) were neutropenia (50%) and thrombocytopenia (21%). Fifteen patients (44%) had ≥1 serious TEAE, including 2 each of febrile neutropenia (related to CC-122), cytokine release syndrome (related to obinutuzumab), and pneumonia. Three deaths occurred during the study (2 PD; 1 AE-related). Overall response rate (ORR) was 59%, including 26% CR and 32% PR (Table 1). Median time to best response was 57 d, and median duration of response was not yet reached. In evaluable patients, 6-mo progression-free survival (PFS) was 63%.

Conclusion
The combination of CC-122 and obinutuzumab was well tolerated and demonstrates promising response rates and durable remissions in R/R patients with B-cell NHL. CC-122 doses of ≥3 mg and obinutuzumab have shown best response rates to date. The study is ongoing to establish the phase II recommended dose.
Session topic: 20. Aggressive Non-Hodgkin lymphoma - Clinical
Keyword(s): Relapse, Obinutuzumab, Non-Hodgkin's lymphoma, B cell lymphoma


