BENDAMUSTINE (B), FOLLOWED BY OBINUTUZUMAB (G, GA101) AND VENETOCLAX (A, ABT-199) IN PATIENTS WITH CHRONIC LYMPHOCYTIC LEUKEMIA (CLL): CLL2-BAG PHASE-II-TRIAL OF THE GERMAN CLL STUDY GROUP (GCLLSG)
(Abstract release date: 05/18/17)
EHA Library. Cramer P. 06/24/17; 181751; S464

Dr. Paula Cramer
Contributions
Contributions
Abstract
Abstract: S464
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 16:45 - 17:00
Location: Hall B
Background
Based on the theoretical “sequential triple-T” concept [Hallek M., Blood 2013; 122(23): 3723-34] of a tailored and targeted treatment aiming for total eradication of minimal residual disease (MRD), the GCLLSG designed the CLL2-BAG trial.
Aims
This prospective, open-label, multicenter phase-II trial investigates a sequential treatment with a B debulking, followed by G and A as induction and maintenance therapy in an all-comer population of physically fit and unfit, treatment-naïve (TN) and relapsed/refractory (R/R) CLL pts.
Methods
Pts with an absolute lymphocyte count (ALC) ≥ 25.000/µl and/or lymph nodes (LN) ≥ 5cm were to receive 2 cycles of B as debulking (70mg/m² d1&2 q28 days), unless contraindicated. In the induction G (1000mg) was administered 3 times in cycle 1 (days 1/2, 8 & 15) and every 4 weeks in cycles 2-6. A was added in cycle 2 with a dose ramp-up (to 400mg daily) over 5 weeks and several safety precautions. In the maintenance therapy, daily intake of A was continued and G administered every 3 months until achievement of a MRD-negative complete response or for up to 24 months.
The primary endpoint is the overall response rate (ORR) at the end of induction therapy; secondary endpoints include MRD evaluations, safety and survival parameters. This primary endpoint analysis is based on uncleaned data, the final analysis will be presented at the meeting
Results
Between May 2015 and January 2016, 66 pts were enrolled. Two R/R pts died of a sepsis and 1 TN pt discontinued due to toxicity during the first induction cycle; these 3 pts with <2 induction cycles were excluded from the analysis as predefined by protocol.
34 pts were treatment-naïve and 29 had R/R CLL (median number of prior therapies: 2, range: 1-8). Median age was 59 (28-77) years, the median CIRS score was 2 (0-14) and 16 pts (25%) had a creatinine clearance of 30-70ml/min. 11 of 59 pts (19%) had a del(17p) and 45 of 61 (74%) had an unmutated IGHV status. Risk categories for TLS at baseline were: low (ALC <25.000/µl & LN <5cm): 9 pts (15%), intermediate (ALC ≥ 25.000/µl or LN 5-10cm): 35 (58%) and high (ALC ≥ 25.000/µl & LN 5-10cm or LN >10cm): 16 (27%), 3 missing.
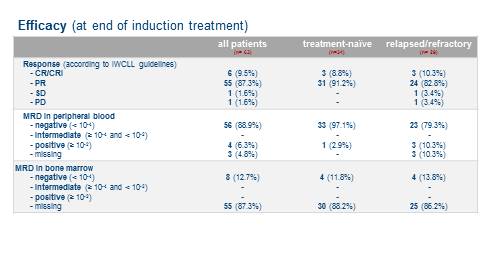
45 pts (71%) received B debulking, 18 (29%) pts immediately started with the induction. 60 pts completed 6 induction cycles with G and A. All TN (100%) and all but two of the R/R pts (93%) responded (table 1); with an ORR of 97% at the end of induction, the primary endpoint was met. MRD negativity (<10-4 by flow cytometry) in peripheral blood (pB) was achieved in 56 pts (89%); MRD assessment from bone marrow was available in 8 pts (4 TN and 4 R/R, among them 4 with a CR and 4 with a PR) and were all negative.
As of January 9th 2017, 83 serious adverse events (SAEs) were reported in 37 pts, including 69 SAEs (83%) related to study treatment. 66 SAEs (80%) were CTC°3-4 and 1 had a fatal outcome (sepsis in 4th induction cycle). Most SAEs occurred in the R/R cohort (61 SAEs, 74%) and during the induction phase (63 SAEs, 76%). Most common SAEs were infections (27 in 16 pts; including 13 CTC°3-5) and hematological disorders (18 in 10 pts; 10 CTC°3-4), followed by infusion-related reactions (6 in 6 pts), laboratory TLS (5 in 5 pts; 1 during B debulking, 1 in induction cycle 1 with G, 2 in cycle 3 and 1 in cycle 4 with G and A) and ischemic coronary artery disorders (5 in 4 pts). No clinical TLS occured.
Conclusion
With an ORR of 97% and a MRD negativity rate of 89% in pB at the end of induction phase this sequential treatment of B debulking, followed by G and A was very efficacious in a heterogeneous study population and well tolerated except for 3 fatal septicaemias in R/R pts.
Session topic: 6. Chronic lymphocytic leukemia and related disorders - Clinical
Keyword(s): Obinutuzumab, MRD, Chronic Lymphocytic Leukemia, bendamustine
Abstract: S464
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 16:45 - 17:00
Location: Hall B
Background
Based on the theoretical “sequential triple-T” concept [Hallek M., Blood 2013; 122(23): 3723-34] of a tailored and targeted treatment aiming for total eradication of minimal residual disease (MRD), the GCLLSG designed the CLL2-BAG trial.
Aims
This prospective, open-label, multicenter phase-II trial investigates a sequential treatment with a B debulking, followed by G and A as induction and maintenance therapy in an all-comer population of physically fit and unfit, treatment-naïve (TN) and relapsed/refractory (R/R) CLL pts.
Methods
Pts with an absolute lymphocyte count (ALC) ≥ 25.000/µl and/or lymph nodes (LN) ≥ 5cm were to receive 2 cycles of B as debulking (70mg/m² d1&2 q28 days), unless contraindicated. In the induction G (1000mg) was administered 3 times in cycle 1 (days 1/2, 8 & 15) and every 4 weeks in cycles 2-6. A was added in cycle 2 with a dose ramp-up (to 400mg daily) over 5 weeks and several safety precautions. In the maintenance therapy, daily intake of A was continued and G administered every 3 months until achievement of a MRD-negative complete response or for up to 24 months.
The primary endpoint is the overall response rate (ORR) at the end of induction therapy; secondary endpoints include MRD evaluations, safety and survival parameters. This primary endpoint analysis is based on uncleaned data, the final analysis will be presented at the meeting
Results
Between May 2015 and January 2016, 66 pts were enrolled. Two R/R pts died of a sepsis and 1 TN pt discontinued due to toxicity during the first induction cycle; these 3 pts with <2 induction cycles were excluded from the analysis as predefined by protocol.
34 pts were treatment-naïve and 29 had R/R CLL (median number of prior therapies: 2, range: 1-8). Median age was 59 (28-77) years, the median CIRS score was 2 (0-14) and 16 pts (25%) had a creatinine clearance of 30-70ml/min. 11 of 59 pts (19%) had a del(17p) and 45 of 61 (74%) had an unmutated IGHV status. Risk categories for TLS at baseline were: low (ALC <25.000/µl & LN <5cm): 9 pts (15%), intermediate (ALC ≥ 25.000/µl or LN 5-10cm): 35 (58%) and high (ALC ≥ 25.000/µl & LN 5-10cm or LN >10cm): 16 (27%), 3 missing.
45 pts (71%) received B debulking, 18 (29%) pts immediately started with the induction. 60 pts completed 6 induction cycles with G and A. All TN (100%) and all but two of the R/R pts (93%) responded (table 1); with an ORR of 97% at the end of induction, the primary endpoint was met. MRD negativity (<10-4 by flow cytometry) in peripheral blood (pB) was achieved in 56 pts (89%); MRD assessment from bone marrow was available in 8 pts (4 TN and 4 R/R, among them 4 with a CR and 4 with a PR) and were all negative.
As of January 9th 2017, 83 serious adverse events (SAEs) were reported in 37 pts, including 69 SAEs (83%) related to study treatment. 66 SAEs (80%) were CTC°3-4 and 1 had a fatal outcome (sepsis in 4th induction cycle). Most SAEs occurred in the R/R cohort (61 SAEs, 74%) and during the induction phase (63 SAEs, 76%). Most common SAEs were infections (27 in 16 pts; including 13 CTC°3-5) and hematological disorders (18 in 10 pts; 10 CTC°3-4), followed by infusion-related reactions (6 in 6 pts), laboratory TLS (5 in 5 pts; 1 during B debulking, 1 in induction cycle 1 with G, 2 in cycle 3 and 1 in cycle 4 with G and A) and ischemic coronary artery disorders (5 in 4 pts). No clinical TLS occured.

Conclusion
With an ORR of 97% and a MRD negativity rate of 89% in pB at the end of induction phase this sequential treatment of B debulking, followed by G and A was very efficacious in a heterogeneous study population and well tolerated except for 3 fatal septicaemias in R/R pts.
Session topic: 6. Chronic lymphocytic leukemia and related disorders - Clinical
Keyword(s): Obinutuzumab, MRD, Chronic Lymphocytic Leukemia, bendamustine
{{ help_message }}
{{filter}}


