
Contributions
Abstract: S459
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 16:45 - 17:00
Location: Hall A
Background
Daratumumab is a human monoclonal antibody targeting CD38 that induces deep and durable responses with significant clinical benefit and is well tolerated as monotherapy and in combination with established standard-of-care regimens in patients with RRMM.
Aims
To provide updated efficacy and safety data from CASTOR, a multicenter, phase 3, randomized, active-controlled study of DVd vs Vd in RRMM.
Methods
Eligible patients with ≥1 prior line of therapy were randomly assigned to 8 cycles (every 3 weeks) of Vd (1.3 mg/m2 SC bortezomib on Days 1, 4, 8, and 11; 20 mg PO/IV dexamethasone on Days 1-2, 4-5, 8-9, and 11-12) with or without daratumumab (16 mg/kg IV once weekly in Cycles 1-3, every 3 weeks for Cycles 4-8, then every 4 weeks until progression). Patients who were refractory to bortezomib were excluded. Progression-free survival (PFS) was the primary endpoint. Minimal residual disease (MRD) was assessed at suspected complete response (CR) and at 6 and 12 months after first dose at 3 sensitivity thresholds (10–4, 10–5, and 10–6) using the ClonoSEQ™ next-generation sequencing (NGS)-based assay (Adaptive Biotechnologies, Seattle, WA).
Results
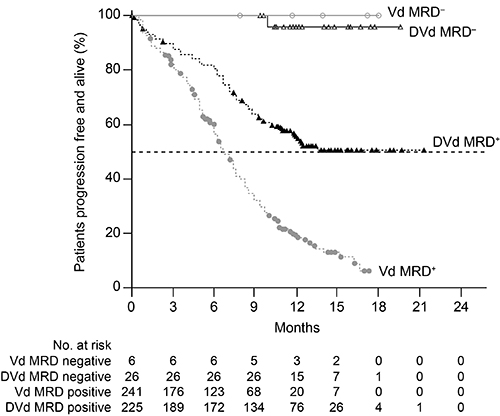
A total of 498 patients were randomized with median (range) age of 64 (30-88) years. Patients received a median (range) of 2 (1-10) prior lines of therapy; 66% of patients previously received bortezomib, and 21% were refractory to lenalidomide in their last prior line of therapy. After median follow-up of 13.0 months, DVd significantly prolonged PFS compared with Vd alone (median: not reached vs 7.1 months; hazard ratio [HR], 0.33; 95% confidence interval [CI], 0.26-0.43; P<0.0001). Twelve-month PFS rates were 60% versus 22%, respectively. Significant PFS benefit was observed with DVd over Vd regardless of the number of prior lines of therapy, although the greatest benefit was seen in patients with 1 prior line of therapy (median: not reached vs 7.9 months; HR, 0.22; 95% CI, 0.14-0.34; P<0.0001). Overall response rate (ORR; 84% vs 63%) and rates of very good partial response (VGPR) or better (62% vs 29%) and CR or better (26% vs 10%) continued to be significantly higher with DVd compared with Vd (P<0.0001 for all). MRD-negative rates were more than 4 times higher at all 3 sensitivity thresholds with DVd versus Vd: 18.3% versus 3.6% at 10–4 (P<0.0001), 10.4% versus 2.4% at 10–5 (P<0.01), and 4.4% versus 0.8% at 10–6 (P<0.01). MRD-negative patients had prolonged PFS compared with MRD-positive patients at 10–5 sensitivity threshold (Figure). At the clinical cut-off date, 37 (15%) deaths in the DVd group and 58 (24%) in the Vd group have been observed (HR, 0.63; 95% CI, 0.42-0.96), and follow up is ongoing. Thrombocytopenia was the most common grade 3 or 4 treatment-emergent adverse event (45% with DVd vs 33% with Vd). No new safety signals were reported after median treatment duration of 11 months with daratumumab. Updated efficacy and safety data with longer follow up will be presented at the meeting.
Conclusion
DVd is superior to Vd in terms of PFS, ORR, depth of response, and MRD-negative rates, with no new safety signals reported. These updated data further support the use of DVd as a standard of care in RRMM, with the greatest benefit observed in patients with 1 prior line of therapy.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Multiple Myeloma, Minimal residual disease (MRD), Immunotherapy, CD38
Abstract: S459
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 16:45 - 17:00
Location: Hall A
Background
Daratumumab is a human monoclonal antibody targeting CD38 that induces deep and durable responses with significant clinical benefit and is well tolerated as monotherapy and in combination with established standard-of-care regimens in patients with RRMM.
Aims
To provide updated efficacy and safety data from CASTOR, a multicenter, phase 3, randomized, active-controlled study of DVd vs Vd in RRMM.
Methods
Eligible patients with ≥1 prior line of therapy were randomly assigned to 8 cycles (every 3 weeks) of Vd (1.3 mg/m2 SC bortezomib on Days 1, 4, 8, and 11; 20 mg PO/IV dexamethasone on Days 1-2, 4-5, 8-9, and 11-12) with or without daratumumab (16 mg/kg IV once weekly in Cycles 1-3, every 3 weeks for Cycles 4-8, then every 4 weeks until progression). Patients who were refractory to bortezomib were excluded. Progression-free survival (PFS) was the primary endpoint. Minimal residual disease (MRD) was assessed at suspected complete response (CR) and at 6 and 12 months after first dose at 3 sensitivity thresholds (10–4, 10–5, and 10–6) using the ClonoSEQ™ next-generation sequencing (NGS)-based assay (Adaptive Biotechnologies, Seattle, WA).
Results
A total of 498 patients were randomized with median (range) age of 64 (30-88) years. Patients received a median (range) of 2 (1-10) prior lines of therapy; 66% of patients previously received bortezomib, and 21% were refractory to lenalidomide in their last prior line of therapy. After median follow-up of 13.0 months, DVd significantly prolonged PFS compared with Vd alone (median: not reached vs 7.1 months; hazard ratio [HR], 0.33; 95% confidence interval [CI], 0.26-0.43; P<0.0001). Twelve-month PFS rates were 60% versus 22%, respectively. Significant PFS benefit was observed with DVd over Vd regardless of the number of prior lines of therapy, although the greatest benefit was seen in patients with 1 prior line of therapy (median: not reached vs 7.9 months; HR, 0.22; 95% CI, 0.14-0.34; P<0.0001). Overall response rate (ORR; 84% vs 63%) and rates of very good partial response (VGPR) or better (62% vs 29%) and CR or better (26% vs 10%) continued to be significantly higher with DVd compared with Vd (P<0.0001 for all). MRD-negative rates were more than 4 times higher at all 3 sensitivity thresholds with DVd versus Vd: 18.3% versus 3.6% at 10–4 (P<0.0001), 10.4% versus 2.4% at 10–5 (P<0.01), and 4.4% versus 0.8% at 10–6 (P<0.01). MRD-negative patients had prolonged PFS compared with MRD-positive patients at 10–5 sensitivity threshold (Figure). At the clinical cut-off date, 37 (15%) deaths in the DVd group and 58 (24%) in the Vd group have been observed (HR, 0.63; 95% CI, 0.42-0.96), and follow up is ongoing. Thrombocytopenia was the most common grade 3 or 4 treatment-emergent adverse event (45% with DVd vs 33% with Vd). No new safety signals were reported after median treatment duration of 11 months with daratumumab. Updated efficacy and safety data with longer follow up will be presented at the meeting.

Conclusion
DVd is superior to Vd in terms of PFS, ORR, depth of response, and MRD-negative rates, with no new safety signals reported. These updated data further support the use of DVd as a standard of care in RRMM, with the greatest benefit observed in patients with 1 prior line of therapy.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Multiple Myeloma, Minimal residual disease (MRD), Immunotherapy, CD38


