
Contributions
Abstract: S423
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 11:45 - 12:00
Location: Hall D
Background
In CML, there is considerable current interest in whether some patients can safely discontinue tyrosine kinase inhibitor (TKI) therapy. However, all studies so far have examined patients in stable MR4 at entry, i.e. BCR-ABL1/ABL1 ratio ≤ 0.01%. Patients in stable major molecular response (MMR) but not MR4 (<0.1 but >0.01%) have not been formally studied; neither have the effects of stepwise TKI withdrawal.
Aims
The present British De-Escalation and Stopping Therapy with Imatinib, Nilotinib or sprYcel (DESTINY) study examines treatment de-escalation as a prelude to complete cessation, in patients in not only stable MR4 but also those with MMR but not MR4.
Methods
Trial entry required first chronic phase of CML, TKI treatment for ≥ 3 years, and either the same TKI (imatinib, dasatinib or nilotinib) since diagnosis or only one switch for intolerance. All PCR tests (minimum of 3) in the 12 months before trial entry must have been ≤0.1% (i.e. MMR), each with ≥ 10,000 ABL1 control transcripts; those with all results ≤ 0.01% were allocated to the ‘stable MR4’ group; the remainder to the ‘MMR but not MR4’ group. Entry criteria were thus virtually identical to the EUROSKI study except that patients with MMR but not MR4 were also separately eligible.
Results
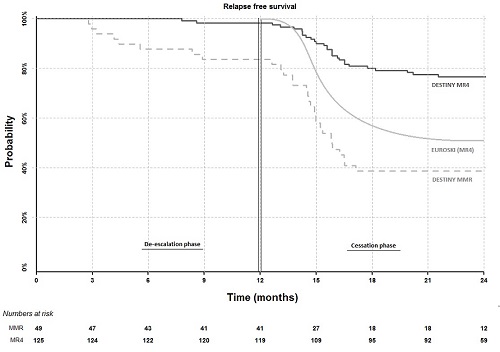
174 patients (male 98; female 76) were recruited after giving informed consent from 20 UK centres. At entry, 148 patients were receiving imatinib, 16 nilotinib and 10 dasatinib, for a median duration of 6.8 years. We reported at ASH 2016 that after 12 months of half-dose therapy, molecular recurrence was lower in patients with stable MR4 at entry (3 of 125 patients; 2.4%) than in those in MMR but not MR4 (9 of 49 patients; 18.4%) (p < 0.001). We now show in the Figure below that during the subsequent 12 months of complete treatment cessation in 117 stable MR4 patients, only 26 further recurrences and 4 withdrawals occurred, giving a recurrence free survival (RFS) of 77% (90% CI: 71-83%) for the overall 24 months for this patient group. The recurrence rate on cessation is higher in the MMR but not MR4 group (20 recurrences and 4 withdrawals among 36 patients during cessation; 39% RFS overall (90% CI: 29-52%); p = < 0.001). In both the stable MR4 group and the MMR but not MR4 groups, no difference in RFS was seen between patients in MR4.5 at entry and those not. In multivariable Cox proportional hazards modelling, addition of the baseline entry PCR result did not add to the predictive effect on RFS of the prior 12 month PCR pattern, whereas the duration of TKI treatment was an additional predictive factor (p = 0.058; HR 0.93), in line with recent data from EUROSKI. The probability of RFS remains unrelated to age, gender, performance status or prior TKI (imatinib vs second generation). No progression to advanced phase was seen; one case lost haematological response.
Conclusion
The present 24 month RFS of 77% for the overall 24 months in patients in stable MR4 appears better than in any comparable study to date, and implies that the initial 12 months of dose reduction may be responsible, perhaps via improved compliance in the few months prior to stopping or through an as yet undefined mechanism.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): Therapy, Chronic myeloid leukemia
Abstract: S423
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 11:45 - 12:00
Location: Hall D
Background
In CML, there is considerable current interest in whether some patients can safely discontinue tyrosine kinase inhibitor (TKI) therapy. However, all studies so far have examined patients in stable MR4 at entry, i.e. BCR-ABL1/ABL1 ratio ≤ 0.01%. Patients in stable major molecular response (MMR) but not MR4 (<0.1 but >0.01%) have not been formally studied; neither have the effects of stepwise TKI withdrawal.
Aims
The present British De-Escalation and Stopping Therapy with Imatinib, Nilotinib or sprYcel (DESTINY) study examines treatment de-escalation as a prelude to complete cessation, in patients in not only stable MR4 but also those with MMR but not MR4.
Methods
Trial entry required first chronic phase of CML, TKI treatment for ≥ 3 years, and either the same TKI (imatinib, dasatinib or nilotinib) since diagnosis or only one switch for intolerance. All PCR tests (minimum of 3) in the 12 months before trial entry must have been ≤0.1% (i.e. MMR), each with ≥ 10,000 ABL1 control transcripts; those with all results ≤ 0.01% were allocated to the ‘stable MR4’ group; the remainder to the ‘MMR but not MR4’ group. Entry criteria were thus virtually identical to the EUROSKI study except that patients with MMR but not MR4 were also separately eligible.
Results
174 patients (male 98; female 76) were recruited after giving informed consent from 20 UK centres. At entry, 148 patients were receiving imatinib, 16 nilotinib and 10 dasatinib, for a median duration of 6.8 years. We reported at ASH 2016 that after 12 months of half-dose therapy, molecular recurrence was lower in patients with stable MR4 at entry (3 of 125 patients; 2.4%) than in those in MMR but not MR4 (9 of 49 patients; 18.4%) (p < 0.001). We now show in the Figure below that during the subsequent 12 months of complete treatment cessation in 117 stable MR4 patients, only 26 further recurrences and 4 withdrawals occurred, giving a recurrence free survival (RFS) of 77% (90% CI: 71-83%) for the overall 24 months for this patient group. The recurrence rate on cessation is higher in the MMR but not MR4 group (20 recurrences and 4 withdrawals among 36 patients during cessation; 39% RFS overall (90% CI: 29-52%); p = < 0.001). In both the stable MR4 group and the MMR but not MR4 groups, no difference in RFS was seen between patients in MR4.5 at entry and those not. In multivariable Cox proportional hazards modelling, addition of the baseline entry PCR result did not add to the predictive effect on RFS of the prior 12 month PCR pattern, whereas the duration of TKI treatment was an additional predictive factor (p = 0.058; HR 0.93), in line with recent data from EUROSKI. The probability of RFS remains unrelated to age, gender, performance status or prior TKI (imatinib vs second generation). No progression to advanced phase was seen; one case lost haematological response.

Conclusion
The present 24 month RFS of 77% for the overall 24 months in patients in stable MR4 appears better than in any comparable study to date, and implies that the initial 12 months of dose reduction may be responsible, perhaps via improved compliance in the few months prior to stopping or through an as yet undefined mechanism.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): Therapy, Chronic myeloid leukemia


