
Contributions
Abstract: S422
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 11:30 - 11:45
Location: Hall D
Background
As safe and effective frontline treatment options for children and adolescents with CML are limited, and no approved therapies exist for patients (pts) resistant/intolerant to imatinib (IM), additional treatment options and alternative formulations are greatly needed for this younger population. Dasatinib (DAS) has proven efficacy in adults with newly diagnosed CML-CP, as well as those resistant/intolerant to IM (Cortes JCO 2016, Shah AJH 2016). Results of a phase 1 study confirmed its dosing and safety in pediatric pts (Zwaan JCO 2013); however, a larger prospective study is necessary to further support the use of DAS in pediatric pts with newly diagnosed or IM-resistant/intolerant CML-CP.
Aims
To determine whether DAS is safe and effective in pediatric pts with CML-CP newly diagnosed or resistant/intolerant to IM enrolled in a phase 2, open-label, nonrandomized prospective clinical trial (CA180-226/NCT00777036).
Methods
Pts aged <18 years were recruited into 3 separate cohorts: (1) IM-resistant/intolerant CML-CP treated with DAS tablets 60 mg/m2 QD, (2) IM-resistant/intolerant CML-AP/BP or Ph+ ALL (enrollment closed early due to poor response), and (3) newly diagnosed CML-CP treated with DAS tablets 60 mg/m2 or DAS 72 mg/m2 powder for oral suspension (PFOS) QD for ≥1 year. PFOS dose was increased by 20% to match the exposure of the tablet in order to maintain a desired efficacy based on the findings from a bioequivalence study in adults. Primary objectives were major cytogenetic response (MCyR) for CML-CP resistant/intolerant to IM and complete cytogenetic response (CCyR) for newly diagnosed CML-CP (MCyR >30% and CCyR >55% considered of clinical interest). Study cohorts were not designed to be comparative.
Results
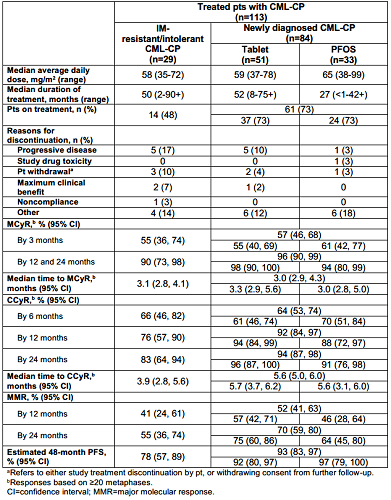
From 145 pts enrolled, 130 were treated; 54% were aged ≥12–<18 years. Within the IM resistant/intolerant group, 25 were resistant, 2 were intolerant, and 2 were undetermined. For pts with CML-CP (n=113), 48% of pts with IM-resistant/intolerant CML-CP and 73% with newly diagnosed CML-CP remained on treatment at the time of this analysis (table). Cumulative rate of MCyR >30% was reached as early as 3 months for IM-resistant/intolerant CML-CP, and a cumulative rate of CCyR >55% was reached as early as 6 months for newly diagnosed CML-CP (table). Estimated progression-free survival (PFS) by 48 months was 78% for IM-resistant/intolerant CML-CP and 93% for newly diagnosed CML-CP (table). Reasons for progression were loss of MCyR (n=3 IM-resistant/intolerant; n=4 newly diagnosed), loss of complete hematologic response (n=2 each), and development of CML-BP (n=2 IM-resistant/intolerant; n=1 newly diagnosed). One death was reported in the IM-resistant/intolerant CML-CP cohort 1 year after stopping DAS (gastrointestinal bleeding). Adverse events (AEs) were consistent with reports in DAS-treated adults, except no DAS-related pleural/pericardial effusion, pulmonary edema/hypertension, or pulmonary arterial hypertension were reported here. Hypersensitivity in a newly diagnosed pt was the only DAS-related AE that led to discontinuation.
Conclusion
Results from the largest prospective and registrational trial of pediatric pts with CML-CP demonstrate that DAS is a safe and effective treatment for pediatric CML-CP. Target responses to first- or second-line that DAS were met as early as 3 and 6 months, respectively, and deep responses were observed. Efficacy and safety of DAS in pediatric pts were similar to those observed in adults; however, unlike in adults, no cases of pleural/pericardial effusion were reported.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): Tyrosine kinase inhibitor, Pediatric, Chronic myeloid leukemia
Abstract: S422
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 11:30 - 11:45
Location: Hall D
Background
As safe and effective frontline treatment options for children and adolescents with CML are limited, and no approved therapies exist for patients (pts) resistant/intolerant to imatinib (IM), additional treatment options and alternative formulations are greatly needed for this younger population. Dasatinib (DAS) has proven efficacy in adults with newly diagnosed CML-CP, as well as those resistant/intolerant to IM (Cortes JCO 2016, Shah AJH 2016). Results of a phase 1 study confirmed its dosing and safety in pediatric pts (Zwaan JCO 2013); however, a larger prospective study is necessary to further support the use of DAS in pediatric pts with newly diagnosed or IM-resistant/intolerant CML-CP.
Aims
To determine whether DAS is safe and effective in pediatric pts with CML-CP newly diagnosed or resistant/intolerant to IM enrolled in a phase 2, open-label, nonrandomized prospective clinical trial (CA180-226/NCT00777036).
Methods
Pts aged <18 years were recruited into 3 separate cohorts: (1) IM-resistant/intolerant CML-CP treated with DAS tablets 60 mg/m2 QD, (2) IM-resistant/intolerant CML-AP/BP or Ph+ ALL (enrollment closed early due to poor response), and (3) newly diagnosed CML-CP treated with DAS tablets 60 mg/m2 or DAS 72 mg/m2 powder for oral suspension (PFOS) QD for ≥1 year. PFOS dose was increased by 20% to match the exposure of the tablet in order to maintain a desired efficacy based on the findings from a bioequivalence study in adults. Primary objectives were major cytogenetic response (MCyR) for CML-CP resistant/intolerant to IM and complete cytogenetic response (CCyR) for newly diagnosed CML-CP (MCyR >30% and CCyR >55% considered of clinical interest). Study cohorts were not designed to be comparative.
Results
From 145 pts enrolled, 130 were treated; 54% were aged ≥12–<18 years. Within the IM resistant/intolerant group, 25 were resistant, 2 were intolerant, and 2 were undetermined. For pts with CML-CP (n=113), 48% of pts with IM-resistant/intolerant CML-CP and 73% with newly diagnosed CML-CP remained on treatment at the time of this analysis (table). Cumulative rate of MCyR >30% was reached as early as 3 months for IM-resistant/intolerant CML-CP, and a cumulative rate of CCyR >55% was reached as early as 6 months for newly diagnosed CML-CP (table). Estimated progression-free survival (PFS) by 48 months was 78% for IM-resistant/intolerant CML-CP and 93% for newly diagnosed CML-CP (table). Reasons for progression were loss of MCyR (n=3 IM-resistant/intolerant; n=4 newly diagnosed), loss of complete hematologic response (n=2 each), and development of CML-BP (n=2 IM-resistant/intolerant; n=1 newly diagnosed). One death was reported in the IM-resistant/intolerant CML-CP cohort 1 year after stopping DAS (gastrointestinal bleeding). Adverse events (AEs) were consistent with reports in DAS-treated adults, except no DAS-related pleural/pericardial effusion, pulmonary edema/hypertension, or pulmonary arterial hypertension were reported here. Hypersensitivity in a newly diagnosed pt was the only DAS-related AE that led to discontinuation.

Conclusion
Results from the largest prospective and registrational trial of pediatric pts with CML-CP demonstrate that DAS is a safe and effective treatment for pediatric CML-CP. Target responses to first- or second-line that DAS were met as early as 3 and 6 months, respectively, and deep responses were observed. Efficacy and safety of DAS in pediatric pts were similar to those observed in adults; however, unlike in adults, no cases of pleural/pericardial effusion were reported.
Session topic: 8. Chronic myeloid leukemia - Clinical
Keyword(s): Tyrosine kinase inhibitor, Pediatric, Chronic myeloid leukemia


