
Contributions
Abstract: S412
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 11:30 - 11:45
Location: Hall B
Background
Nivolumab, a fully human IgG4 monoclonal antibody targeting programmed death-1, is an immune checkpoint inhibitor that augments T-cell activation and antitumor responses. Nivolumab is indicated for pts with relapsed/refractory (RR) classical Hodgkin lymphoma (cHL) following autologous stem cell transplantation (ASCT) and brentuximab vedotin (BV) treatment. The multicohort phase 2 CheckMate 205 trial (NCT02181738) enrolled pts with RR cHL after ASCT. Initial analyses revealed high objective response rates (ORR), encouraging duration of response (DOR) and an acceptable safety profile (Younes A et al, Lancet Oncol 2016). Durable responses to therapy are valuable in pts with progressive disease after failure of ASCT due to their limited treatment options.
Aims
To report extended follow-up data for all pts with RR cHL after failure of ASCT in CheckMate 205.
Methods
This single-arm multicenter trial enrolled pts (age ≥18 y) with RR cHL after ASCT into 1 of 3 independent cohorts (Cohort A: BV-naïve; Cohort B: BV only after ASCT; Cohort C: BV before and/or after ASCT). All pts received nivolumab 3 mg/kg every 2 wk until disease progression or unacceptable toxicity. Pts in Cohort C with a persistent complete response (CR) for 1 y were to discontinue nivolumab and could resume at relapse. Primary endpoint was ORR per Independent Radiology Review Committee. Secondary endpoints included DOR; progression-free survival (PFS), overall survival (OS), and safety were exploratory endpoints. All pts provided written informed consent.
Results
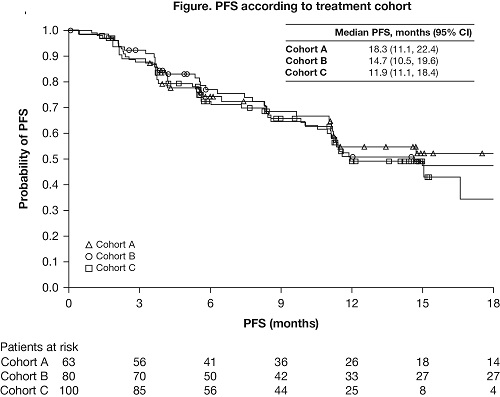
In total, 243 pts were treated: 63 in Cohort A (BV-naïve), 80 in Cohort B (BV after ASCT), and 100 in Cohort C (BV before [n=33], after [n=58], or before and after [n=9] ASCT). Median (range) age was 34 (18–72) y and 77% of pts had advanced (stage III+) disease at study entry. BV-naïve pts had fewer prior lines of therapy (median of 2 vs 4 with prior BV). At Dec 2016 database lock, median (min, max) follow-up was 19 (1, 25), 23 (2, 27) and 16 (1, 20) mo in Cohorts A, B, and C, respectively. Overall, 40% of pts were still on treatment; the most common reason for discontinuation was disease progression (26%). ORR was 65% in Cohort A, 68% in Cohort B, and 73% in Cohort C, with 29%, 13%, and 12% CR, respectively. Median (95% CI) DOR was 20 (13, 20), 16 (8, 20), and 15 (9, 17) mo in Cohorts A, B, and C, respectively. DOR for patients with CR was 20 months for BV-naïve patients (Cohort A) and ≥15 mo for BV-treated patients (Cohorts B and C); DOR for patients with partial response (PR) was 17 and ≥11 months, respectively. PFS by cohort is shown (Figure). Prolonged median PFS was seen for patients with CR (≥17 mo in each cohort), PR (≥15 mo in each cohort), and stable disease (≥9 mo in each cohort). Median OS was not reached in any cohort. The most common drug-related AEs were fatigue (23%), diarrhea (15%), infusion reactions (IRs; 14%), and rash (12%); grade 3–4 drug-related AEs in ≥3% of pts were lipase increases (5%), alanine aminotransferase increases (3%), and neutropenia (3%). The most common drug-related serious AEs were IRs (2%) and pneumonitis (1%). To facilitate translation to practice, efficacy results by sequencing of prior BV treatment will be presented.
Conclusion
With extended follow-up, high and durable rates of CR and PR to nivolumab therapy were observed in pts with RR cHL after ASCT, irrespective of BV treatment history.
Session topic: 17. Hodgkin lymphoma - Clinical
Keyword(s): Monoclonal antibody, Immunotherapy, Hodgkin's Lymphoma, Clinical Trial
Abstract: S412
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 11:30 - 11:45
Location: Hall B
Background
Nivolumab, a fully human IgG4 monoclonal antibody targeting programmed death-1, is an immune checkpoint inhibitor that augments T-cell activation and antitumor responses. Nivolumab is indicated for pts with relapsed/refractory (RR) classical Hodgkin lymphoma (cHL) following autologous stem cell transplantation (ASCT) and brentuximab vedotin (BV) treatment. The multicohort phase 2 CheckMate 205 trial (NCT02181738) enrolled pts with RR cHL after ASCT. Initial analyses revealed high objective response rates (ORR), encouraging duration of response (DOR) and an acceptable safety profile (Younes A et al, Lancet Oncol 2016). Durable responses to therapy are valuable in pts with progressive disease after failure of ASCT due to their limited treatment options.
Aims
To report extended follow-up data for all pts with RR cHL after failure of ASCT in CheckMate 205.
Methods
This single-arm multicenter trial enrolled pts (age ≥18 y) with RR cHL after ASCT into 1 of 3 independent cohorts (Cohort A: BV-naïve; Cohort B: BV only after ASCT; Cohort C: BV before and/or after ASCT). All pts received nivolumab 3 mg/kg every 2 wk until disease progression or unacceptable toxicity. Pts in Cohort C with a persistent complete response (CR) for 1 y were to discontinue nivolumab and could resume at relapse. Primary endpoint was ORR per Independent Radiology Review Committee. Secondary endpoints included DOR; progression-free survival (PFS), overall survival (OS), and safety were exploratory endpoints. All pts provided written informed consent.
Results
In total, 243 pts were treated: 63 in Cohort A (BV-naïve), 80 in Cohort B (BV after ASCT), and 100 in Cohort C (BV before [n=33], after [n=58], or before and after [n=9] ASCT). Median (range) age was 34 (18–72) y and 77% of pts had advanced (stage III+) disease at study entry. BV-naïve pts had fewer prior lines of therapy (median of 2 vs 4 with prior BV). At Dec 2016 database lock, median (min, max) follow-up was 19 (1, 25), 23 (2, 27) and 16 (1, 20) mo in Cohorts A, B, and C, respectively. Overall, 40% of pts were still on treatment; the most common reason for discontinuation was disease progression (26%). ORR was 65% in Cohort A, 68% in Cohort B, and 73% in Cohort C, with 29%, 13%, and 12% CR, respectively. Median (95% CI) DOR was 20 (13, 20), 16 (8, 20), and 15 (9, 17) mo in Cohorts A, B, and C, respectively. DOR for patients with CR was 20 months for BV-naïve patients (Cohort A) and ≥15 mo for BV-treated patients (Cohorts B and C); DOR for patients with partial response (PR) was 17 and ≥11 months, respectively. PFS by cohort is shown (Figure). Prolonged median PFS was seen for patients with CR (≥17 mo in each cohort), PR (≥15 mo in each cohort), and stable disease (≥9 mo in each cohort). Median OS was not reached in any cohort. The most common drug-related AEs were fatigue (23%), diarrhea (15%), infusion reactions (IRs; 14%), and rash (12%); grade 3–4 drug-related AEs in ≥3% of pts were lipase increases (5%), alanine aminotransferase increases (3%), and neutropenia (3%). The most common drug-related serious AEs were IRs (2%) and pneumonitis (1%). To facilitate translation to practice, efficacy results by sequencing of prior BV treatment will be presented.

Conclusion
With extended follow-up, high and durable rates of CR and PR to nivolumab therapy were observed in pts with RR cHL after ASCT, irrespective of BV treatment history.
Session topic: 17. Hodgkin lymphoma - Clinical
Keyword(s): Monoclonal antibody, Immunotherapy, Hodgkin's Lymphoma, Clinical Trial


