
Contributions
Abstract: S410
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 12:15 - 12:30
Location: Hall A
Background
Previous phase I-II studies showed that Carfilzomib-Lenalidomide-Dexamethasone (KRd) and Carfilzomib-Cyclophosphamide-Dexamethasone (KCd) combinations are safe and effective in patients with newly diagnosed multiple myeloma (NDMM) (Jakubowiak Blood 2012, Bringhen Blood 2014).
Aims
The FORTE trial compared KCd vs KRd in transplant-eligible patients. Here we report results of the first planned safety interim analysis on induction and mobilization, and preliminary efficacy data.
Methods
NDMM patients younger than 65 years of age were included. Patients were randomized (1:1:1; stratification ISS and age) to: 4 28-day KCd cycles (carfilzomib:20/36 mg/m2 IV d 1, 2, 8, 9, 15, 16; cyclophosphamide 300 mg/m2 d 1, 8, 15; dexamethasone: 20 mg d 1, 2, 8, 9, 15, 16) followed by high-dose melphalan and autologous stem cell transplantation (MEL200-ASCT) and consolidation with 4 KCd cycles; or 4 28-day KRd cycles (carfilzomib and dexamethasone as above; lenalidomide:25 mg d 1-21) followed by MEL200-ASCT and 4 KRd cycles; or 12 KRd cycles. After the 4th induction cycle, all patients received Cyclophosphamide 2 g/m2, followed by peripheral blood stem cell collection. For the present interim analysis, we pooled together data of the two KRd groups, because patients in the two groups in fact received the same treatment until mobilization. Data cut-off was October 30, 2016.
Results
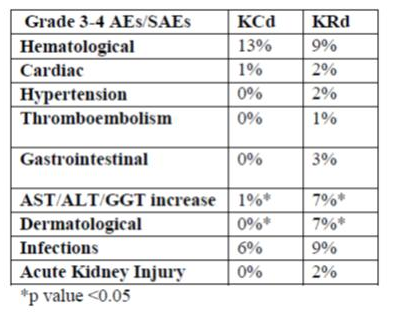
A total of 281 patients were evaluated (94 assigned to KCd treatment and 187 to KRd treatment). The most frequent grade 3-4 adverse events (AEs) and serious AEs (SAEs) in both arms were hematological (mainly neutropenia) and infections (mainly pneumonia/fever); increased AST/ALT/GGT (mainly reversible) and dermatological (rash) AEs were more frequent among KRd patients; cardiac AEs were 2% with KRd (including atrial fibrillation [1%] and ischemic heart disease [1%]) vs 1% with KCd (atrial fibrillation). Death occurred in 1 patient in the KCd group (infection not treatment-related) vs 3 patients in the KRd group (2 cardiac arrest [1 not treatment-related], 1 infection not treatment-related). In the KCd vs KRd arms, 99% vs 95% (P=0.44) of pts mobilized stem cells (median number of PBSC collected: 9 vs 6x10^6CD34/Kg with KCd vs KRd). Plerixafor was required in 10% vs 24% (P=0.01), respectively. At least a very good partial response (VGPR) was reported in 61% of patients receiving KCd vs 74% receiving KRd (P=0.05).
Conclusion
Safety profile was acceptable; more patients required plerixafor in the KRd arm. Rate of VGPR was higher with KRd. Updated data on a higher number of patients will be presented at the meeting. The trial is registered at Clinicaltrials.gov: NCT02203643
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Transplant, Proteasome inhibitor, Multiple Myeloma, Imids
Abstract: S410
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 12:15 - 12:30
Location: Hall A
Background
Previous phase I-II studies showed that Carfilzomib-Lenalidomide-Dexamethasone (KRd) and Carfilzomib-Cyclophosphamide-Dexamethasone (KCd) combinations are safe and effective in patients with newly diagnosed multiple myeloma (NDMM) (Jakubowiak Blood 2012, Bringhen Blood 2014).
Aims
The FORTE trial compared KCd vs KRd in transplant-eligible patients. Here we report results of the first planned safety interim analysis on induction and mobilization, and preliminary efficacy data.
Methods
NDMM patients younger than 65 years of age were included. Patients were randomized (1:1:1; stratification ISS and age) to: 4 28-day KCd cycles (carfilzomib:20/36 mg/m2 IV d 1, 2, 8, 9, 15, 16; cyclophosphamide 300 mg/m2 d 1, 8, 15; dexamethasone: 20 mg d 1, 2, 8, 9, 15, 16) followed by high-dose melphalan and autologous stem cell transplantation (MEL200-ASCT) and consolidation with 4 KCd cycles; or 4 28-day KRd cycles (carfilzomib and dexamethasone as above; lenalidomide:25 mg d 1-21) followed by MEL200-ASCT and 4 KRd cycles; or 12 KRd cycles. After the 4th induction cycle, all patients received Cyclophosphamide 2 g/m2, followed by peripheral blood stem cell collection. For the present interim analysis, we pooled together data of the two KRd groups, because patients in the two groups in fact received the same treatment until mobilization. Data cut-off was October 30, 2016.
Results
A total of 281 patients were evaluated (94 assigned to KCd treatment and 187 to KRd treatment). The most frequent grade 3-4 adverse events (AEs) and serious AEs (SAEs) in both arms were hematological (mainly neutropenia) and infections (mainly pneumonia/fever); increased AST/ALT/GGT (mainly reversible) and dermatological (rash) AEs were more frequent among KRd patients; cardiac AEs were 2% with KRd (including atrial fibrillation [1%] and ischemic heart disease [1%]) vs 1% with KCd (atrial fibrillation). Death occurred in 1 patient in the KCd group (infection not treatment-related) vs 3 patients in the KRd group (2 cardiac arrest [1 not treatment-related], 1 infection not treatment-related). In the KCd vs KRd arms, 99% vs 95% (P=0.44) of pts mobilized stem cells (median number of PBSC collected: 9 vs 6x10^6CD34/Kg with KCd vs KRd). Plerixafor was required in 10% vs 24% (P=0.01), respectively. At least a very good partial response (VGPR) was reported in 61% of patients receiving KCd vs 74% receiving KRd (P=0.05).

Conclusion
Safety profile was acceptable; more patients required plerixafor in the KRd arm. Rate of VGPR was higher with KRd. Updated data on a higher number of patients will be presented at the meeting. The trial is registered at Clinicaltrials.gov: NCT02203643
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Transplant, Proteasome inhibitor, Multiple Myeloma, Imids


