
Contributions
Abstract: S407
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 11:30 - 11:45
Location: Hall A
Background
Combining anti-myeloma induction therapies limits the impact of clonal heterogeneity on resistance to therapy, maximising response and associated clinical outcomes. Triplet combinations induce deeper, longer remissions than doublets and those containing an immunomodulatory agent, a proteasome inhibitor (PI) or both are the current standard of care in Europe/US. Potential approaches to further improve outcomes include response-adapted induction, treating suboptimal responders with sequential treatment using an agent with a different mechanism of action, or intensifying induction for all patients by the use of quadruplet combinations upfront.
Aims
The UK NCRI Myeloma XI trial is a large, phase III study comparing, in transplant eligible (TE) patients, the induction quadruplet carfilzomib, cyclophosphamide, lenalidomide and dexamethasone (KCRD) to the sequential strategy of triplet immunomodulatory combinations (with thalidomide or lenalidomide) followed by additional pre-transplant consolidation with PI triplet therapy for those with a suboptimal response.
Methods
In 2013, the TE pathway of the Myeloma XI study was amended to include KCRD given in 28 day cycles (carfilzomib 36mg/m2 IV d1-2,8-9,15-16 (20mg/m2 #1d1-2), cyclophosphamide (cyclo) 500mg PO d1,8, lenalidomide (len) 25mg PO d1-21, dexamethasone (dex) 40mg PO d1-4,8-9,15-16). Patients were randomised to this up-front quadruplet or the sequential strategy of CRD (cyclo 500mg PO d1,8, len 25mg PO d1-21 PO daily, dex 40mg PO d1-4, 12-15) or CTD (cyclo 500mg PO d1,8,15 thalidomide 100-200mg PO daily, dex 40mg PO d1-4,12-15) given to max. response. Patients with VGPR/CR proceeded straight to ASCT, those with PR/MR were randomised to sequential CVD (cyclo 500mg d1,8,15, bortezomib 1.3mg/m2 IV/SC d1,4,8,11, dex 20mg PO d1,2,4,5,8,9,11,12) or nothing and those with SD/PD all received sequential CVD. At day 100 post ASCT there was a maintenance randomisation between lenalidomide and observation. The trial has now closed to recruitment and all patients have completed induction therapy. This analysis compares responses and toxicity of the different regimens.
Results
2568 TE patients underwent induction randomisation (CTD 1021, CRD 1021, KCRD 526). Patients were comparable with respect to age (median 59 years), sex and other key laboratory parameters. Patients were mandated to receive a minimum of 4 cycles of initial induction with therapy continued to maximum response. The median number of cycles delivered was CTD: 5, CRD: 5, KCRD: 4. Grade ≥3 haematological toxicities differed between the groups. (Neutropenia CTD: 12%, CRD: 22%, KCRD: 16%; Thrombocytopenia CTD: 3.4%, CRD: 4.5%, KCRD: 8.1%; Anaemia CTD: 6.7%, CRD: 9.6%, KCRD 10%). Grade ≥2 neurological toxicity was greater with the thalidomide-containing regimen (Sensory neuropathy CTD: 9.5%, CRD: 3.4%, KCRD: 2.3%). There was no statistically significant difference in rates of investigator reported, all-grade, thromboembolic events between regimens (CTD: 11.8%, CRD 11.1%, KCRD 14.7%).
Conclusion
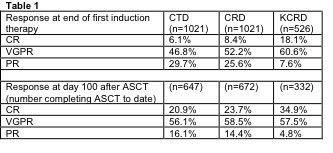
Induction therapy with KCRD, an outpatient delivered quadruplet regimen, was associated with deeper responses than immunomodulatory triplet therapy (CRD/CTD) and was well tolerated. Deeper responses persisted after ASCT, with an impressive response rate ≥VGPR of 92% with KCRD.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Proteasome inhibitor, Myeloma, Induction chemotherapy, Immunomodulatory thalidomide analog
Abstract: S407
Type: Oral Presentation
Presentation during EHA22: On Saturday, June 24, 2017 from 11:30 - 11:45
Location: Hall A
Background
Combining anti-myeloma induction therapies limits the impact of clonal heterogeneity on resistance to therapy, maximising response and associated clinical outcomes. Triplet combinations induce deeper, longer remissions than doublets and those containing an immunomodulatory agent, a proteasome inhibitor (PI) or both are the current standard of care in Europe/US. Potential approaches to further improve outcomes include response-adapted induction, treating suboptimal responders with sequential treatment using an agent with a different mechanism of action, or intensifying induction for all patients by the use of quadruplet combinations upfront.
Aims
The UK NCRI Myeloma XI trial is a large, phase III study comparing, in transplant eligible (TE) patients, the induction quadruplet carfilzomib, cyclophosphamide, lenalidomide and dexamethasone (KCRD) to the sequential strategy of triplet immunomodulatory combinations (with thalidomide or lenalidomide) followed by additional pre-transplant consolidation with PI triplet therapy for those with a suboptimal response.
Methods
In 2013, the TE pathway of the Myeloma XI study was amended to include KCRD given in 28 day cycles (carfilzomib 36mg/m2 IV d1-2,8-9,15-16 (20mg/m2 #1d1-2), cyclophosphamide (cyclo) 500mg PO d1,8, lenalidomide (len) 25mg PO d1-21, dexamethasone (dex) 40mg PO d1-4,8-9,15-16). Patients were randomised to this up-front quadruplet or the sequential strategy of CRD (cyclo 500mg PO d1,8, len 25mg PO d1-21 PO daily, dex 40mg PO d1-4, 12-15) or CTD (cyclo 500mg PO d1,8,15 thalidomide 100-200mg PO daily, dex 40mg PO d1-4,12-15) given to max. response. Patients with VGPR/CR proceeded straight to ASCT, those with PR/MR were randomised to sequential CVD (cyclo 500mg d1,8,15, bortezomib 1.3mg/m2 IV/SC d1,4,8,11, dex 20mg PO d1,2,4,5,8,9,11,12) or nothing and those with SD/PD all received sequential CVD. At day 100 post ASCT there was a maintenance randomisation between lenalidomide and observation. The trial has now closed to recruitment and all patients have completed induction therapy. This analysis compares responses and toxicity of the different regimens.
Results
2568 TE patients underwent induction randomisation (CTD 1021, CRD 1021, KCRD 526). Patients were comparable with respect to age (median 59 years), sex and other key laboratory parameters. Patients were mandated to receive a minimum of 4 cycles of initial induction with therapy continued to maximum response. The median number of cycles delivered was CTD: 5, CRD: 5, KCRD: 4. Grade ≥3 haematological toxicities differed between the groups. (Neutropenia CTD: 12%, CRD: 22%, KCRD: 16%; Thrombocytopenia CTD: 3.4%, CRD: 4.5%, KCRD: 8.1%; Anaemia CTD: 6.7%, CRD: 9.6%, KCRD 10%). Grade ≥2 neurological toxicity was greater with the thalidomide-containing regimen (Sensory neuropathy CTD: 9.5%, CRD: 3.4%, KCRD: 2.3%). There was no statistically significant difference in rates of investigator reported, all-grade, thromboembolic events between regimens (CTD: 11.8%, CRD 11.1%, KCRD 14.7%).

Conclusion
Induction therapy with KCRD, an outpatient delivered quadruplet regimen, was associated with deeper responses than immunomodulatory triplet therapy (CRD/CTD) and was well tolerated. Deeper responses persisted after ASCT, with an impressive response rate ≥VGPR of 92% with KCRD.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Proteasome inhibitor, Myeloma, Induction chemotherapy, Immunomodulatory thalidomide analog


