
Contributions
Abstract: S111
Type: Oral Presentation
Presentation during EHA22: On Friday, June 23, 2017 from 11:45 - 12:00
Location: Hall C
Background
A comprehensive AML risk assessment, based on the integration of cytogenetic/genetic data and minimal residual disease (MRD) status, can help optimize patients' (pts) therapeutic post-remission allocation.
Aims
To evaluate the feasibility and results of a phase II trial of intensive chemotherapy in which risk-assignment and post-remission therapy of young patients with AML was based on pre-treatment cytogenetic/genetic data and post-consolidation levels of MRD.
Methods
Results
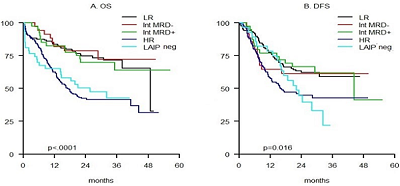
Conclusion
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): MRD, Molecular markers, Cytogenetics, AML
Abstract: S111
Type: Oral Presentation
Presentation during EHA22: On Friday, June 23, 2017 from 11:45 - 12:00
Location: Hall C
Background
A comprehensive AML risk assessment, based on the integration of cytogenetic/genetic data and minimal residual disease (MRD) status, can help optimize patients' (pts) therapeutic post-remission allocation.
Aims
To evaluate the feasibility and results of a phase II trial of intensive chemotherapy in which risk-assignment and post-remission therapy of young patients with AML was based on pre-treatment cytogenetic/genetic data and post-consolidation levels of MRD.
Methods
Results

Conclusion
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): MRD, Molecular markers, Cytogenetics, AML


