
Contributions
Abstract: S110
Type: Oral Presentation
Presentation during EHA22: On Friday, June 23, 2017 from 11:30 - 11:45
Location: Hall C
Background
Mutations in Fms-like tyrosine kinase 3 (FLT3) are common in patients with acute myeloid leukemia (AML) and are associated with an aggressive disease course and a poor prognosis. Notably, FLT3 internal tandem duplications (ITD) predict early relapse and short overall survival (OS) after chemotherapy. Gilteritinib, a highly selective FLT3/AXL inhibitor, has displayed antileukemic activity in FLT3 mutation-positive (FLT3mut+) relapsed/refractory (R/R) AML in the CHRYSALIS Phase 1/2 study (NCT02014558), specifically at doses ≥80 mg/d.
Aims
To assess molecular response to gilteritinib in a CHRYSALIS subpopulation.
Methods
This exploratory analysis evaluated molecular response in patients aged ≥18 years with FLT3mut+ R/R AML who had been treated with 120 or 200 mg/d gilteritinib. These doses were identified due to their ability to induce high clinical response rates, and consistent, potent FLT3 inhibition in correlative assays. Molecular response was assessed in patients who had bone marrow aspirates obtained at baseline and at ≥1 additional time point. FLT3-ITD and total FLT3 were quantified by next-generation sequencing to assess molecular response. A Cox regression model of OS by Kaplan-Meier estimation established a FLT3-ITD:total FLT3 ratio (ITD signal ratio) of 10−2 as the threshold for improved survival.
Results
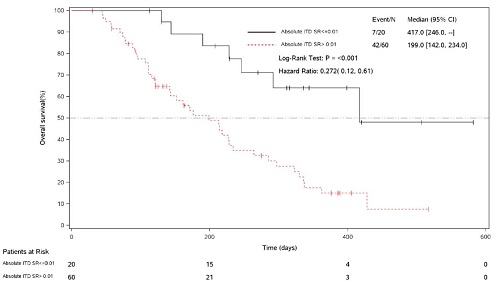
Of the 147 FLT3-ITDmut+ patients who had received gilteritinib 120 or 200 mg/d, 80 patients had bone marrow aspirates at baseline and at ≥1 additional time point, and were included in this analysis. The composite response rate (defined as CR plus CRi plus CRp) for these 80 patients was 55%. During response, 20 patients (25%) had an ITD signal ratio of ≤10−2. Of these 20 patients, 18 had an ITD signal ratio of ≤10−3 (major molecular response [MMR]) and 13 had an ITD signal ratio of ≤10−4 (minimal residual disease [MRD] negative). The median time to achieve minimum ITD signal ratio was 54 days. Elimination of morphologic leukemia was observed in 80% of patients with ITD signal ratios <10−2. Patients who had an ITD signal ratio ≤10−2, MMR, or were MRD negative had significantly longer median OS than those who did not (Table; Figure).
Molecular Response | Achieved a Molecular Response | Did not Achieve a Molecular Response | P-value | ||
n | Median OS, Days (95% CI) | n | Median OS, Days (95% CI) | ||
ITD signal ratio ≤10−2 | 20 | 417 (246–NA) | 60 | 199 (142–234) | <.001 |
MMR | 18 | 417 (228–NA) | 62 | 213 (143–264) | .003 |
MRD negative | 13 | 417 (228–NA) | 67 | 213 (144–264) | .002 |
Conclusion
Molecular responses to gilteritinib in FLT3-ITDmut+ R/R AML correlated with clinical response and improved OS. This is the first demonstration of a robust molecular response to a FLT3 inhibitor in AML. These data suggest that the ITD signal ratio may predict a durable clinical benefit of gilteritinib therapy and validate FLT3 as a critical therapeutic target in AML.
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): Acute Myeloid Leukemia, Molecular response, FLT3
Abstract: S110
Type: Oral Presentation
Presentation during EHA22: On Friday, June 23, 2017 from 11:30 - 11:45
Location: Hall C
Background
Mutations in Fms-like tyrosine kinase 3 (FLT3) are common in patients with acute myeloid leukemia (AML) and are associated with an aggressive disease course and a poor prognosis. Notably, FLT3 internal tandem duplications (ITD) predict early relapse and short overall survival (OS) after chemotherapy. Gilteritinib, a highly selective FLT3/AXL inhibitor, has displayed antileukemic activity in FLT3 mutation-positive (FLT3mut+) relapsed/refractory (R/R) AML in the CHRYSALIS Phase 1/2 study (NCT02014558), specifically at doses ≥80 mg/d.
Aims
To assess molecular response to gilteritinib in a CHRYSALIS subpopulation.
Methods
This exploratory analysis evaluated molecular response in patients aged ≥18 years with FLT3mut+ R/R AML who had been treated with 120 or 200 mg/d gilteritinib. These doses were identified due to their ability to induce high clinical response rates, and consistent, potent FLT3 inhibition in correlative assays. Molecular response was assessed in patients who had bone marrow aspirates obtained at baseline and at ≥1 additional time point. FLT3-ITD and total FLT3 were quantified by next-generation sequencing to assess molecular response. A Cox regression model of OS by Kaplan-Meier estimation established a FLT3-ITD:total FLT3 ratio (ITD signal ratio) of 10−2 as the threshold for improved survival.
Results
Of the 147 FLT3-ITDmut+ patients who had received gilteritinib 120 or 200 mg/d, 80 patients had bone marrow aspirates at baseline and at ≥1 additional time point, and were included in this analysis. The composite response rate (defined as CR plus CRi plus CRp) for these 80 patients was 55%. During response, 20 patients (25%) had an ITD signal ratio of ≤10−2. Of these 20 patients, 18 had an ITD signal ratio of ≤10−3 (major molecular response [MMR]) and 13 had an ITD signal ratio of ≤10−4 (minimal residual disease [MRD] negative). The median time to achieve minimum ITD signal ratio was 54 days. Elimination of morphologic leukemia was observed in 80% of patients with ITD signal ratios <10−2. Patients who had an ITD signal ratio ≤10−2, MMR, or were MRD negative had significantly longer median OS than those who did not (Table; Figure).
Molecular Response | Achieved a Molecular Response | Did not Achieve a Molecular Response | P-value | ||
n | Median OS, Days (95% CI) | n | Median OS, Days (95% CI) | ||
ITD signal ratio ≤10−2 | 20 | 417 (246–NA) | 60 | 199 (142–234) | <.001 |
MMR | 18 | 417 (228–NA) | 62 | 213 (143–264) | .003 |
MRD negative | 13 | 417 (228–NA) | 67 | 213 (144–264) | .002 |

Conclusion
Molecular responses to gilteritinib in FLT3-ITDmut+ R/R AML correlated with clinical response and improved OS. This is the first demonstration of a robust molecular response to a FLT3 inhibitor in AML. These data suggest that the ITD signal ratio may predict a durable clinical benefit of gilteritinib therapy and validate FLT3 as a critical therapeutic target in AML.
Session topic: 4. Acute myeloid leukemia - Clinical
Keyword(s): Acute Myeloid Leukemia, Molecular response, FLT3


