
Contributions
Abstract: S107
Type: Oral Presentation
Presentation during EHA22: On Friday, June 23, 2017 from 12:00 - 12:15
Location: Hall B
Background
Intravenous (IV) rituximab plus chemotherapy is standard treatment for diffuse large B-cell lymphoma (DLBCL). A subcutaneous (SC) formulation of rituximab may simplify treatment and reduce burden.
Aims
MabEase (NCT01649856) studied efficacy, safety and patient (pt) satisfaction with rituximab SC or IV plus CHOP as first-line DLBCL treatment.
Methods
Pts were randomized 2:1 to rituximab SC (IV 375mg/m2 cycle 1; SC 1400mg cycles 2–8) or IV (375mg/m2 cycles 1–8) plus CHOP every 14 or 21 days. The primary endpoint was investigator-assessed complete response (CR)/unconfirmed CR (CRu) at the end of induction (EOI). Secondary endpoints included safety, survival, treatment satisfaction (Cancer Treatment Satisfaction Questionnaire [CTSQ], Rituximab Administration Satisfaction Questionnaire [RASQ]) and time savings. Follow-up continued until at least 24 months after EOI in the last patient recruited.
Results
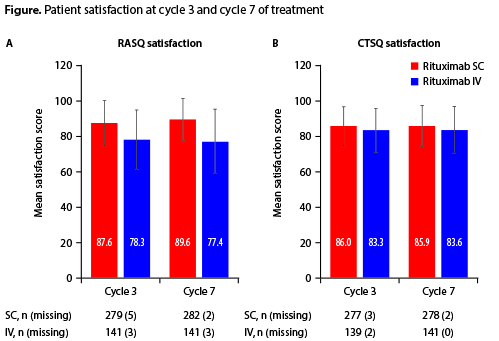
Of 576 pts (381 SC; 195 IV), 572 (378 SC; 194 IV) received treatment. EOI CR/CRu rates were 50.6% (95% CI 45.3–55.9) and 42.4 (95% CI 35.1–49.7) in the SC and IV groups, respectively (Table). After 35 months’ median follow-up, median progression-free survival (PFS), event-free survival (EFS) and overall survival (OS) were not reached in either arm and no statistically significant differences were observed between treatment arms. PFS, EFS and OS rates were also similar at 24 months’ follow-up (non-significant differences; Table). Grade ³3 adverse events (58.3% SC; 54.3% IV) and administration-related reactions (21% in both groups) were similar between arms. Of SC recipients, 5.7% had injection site reactions vs none in the IV group (p<0.001). Febrile neutropenia occurred more often in the SC arm (12.5% vs 6.9% in IV, p=0.06). RASQ scores for ‘impact on activities of daily living’, ‘convenience’ and ‘satisfaction’ were improved with SC vs IV; CTSQ scores were similar between arms (Figure). When pts in the SC group were asked, if given the option, which treatment they would prefer, 90.8% stated a preference for SC over IV. Median administration time (6 minutes SC vs 2.6–3.0 hours IV) and chair/bed and overall hospital times were shorter with SC than with IV treatment.
Table. Efficacy endpoints in the intent-to-treat population | ||||
Efficacy endpoint | Rituximab SC plus CHOP | Rituximab IV plus CHOP | ||
N | % (95% CI) | N | % (95% CI) | |
End of induction treatment | ||||
CR/CRu | 342 | 50.6 (45.3–55.9) | 177 | 42.4 (35.1–49.7) |
PR | 342 | 31.6 (26.7–36.8) | 177 | 35.6 (28.6–43.1) |
PD | 342 | 3.8 (2.0–6.4) | 177 | 6.2 (3.1–10.8) |
ORR | 342 | 82.2 (77.7–86.1) | 177 | 78.0 (71.1–83.8) |
24 months’ follow-up | ||||
PFS* | 342 | 67.5 (62.6–72.5) | 177 | 72.3 (65.7–78.9) |
EFS† | 342 | 61.7 (56.5–66.8) | 177 | 66.7 (59.7–73.6) |
OS | 342 | 87.4 (83.2–90.5) | 177 | 88.0 (82.0–92.1) |
*p=0.264. †p=0.265. ORR, overall response rate; PD, progressive disease; PR, partial response. | ||||
Conclusion
Rituximab SC had similar efficacy and safety to the IV form, with improvements in patient satisfaction ratings, and administration/hospital time savings. Our findings support the use of rituximab SC in this setting.
Session topic: 20. Aggressive Non-Hodgkin lymphoma - Clinical
Keyword(s): Diffuse large B cell lymphoma, CHOP, Rituximab
Abstract: S107
Type: Oral Presentation
Presentation during EHA22: On Friday, June 23, 2017 from 12:00 - 12:15
Location: Hall B
Background
Intravenous (IV) rituximab plus chemotherapy is standard treatment for diffuse large B-cell lymphoma (DLBCL). A subcutaneous (SC) formulation of rituximab may simplify treatment and reduce burden.
Aims
MabEase (NCT01649856) studied efficacy, safety and patient (pt) satisfaction with rituximab SC or IV plus CHOP as first-line DLBCL treatment.
Methods
Pts were randomized 2:1 to rituximab SC (IV 375mg/m2 cycle 1; SC 1400mg cycles 2–8) or IV (375mg/m2 cycles 1–8) plus CHOP every 14 or 21 days. The primary endpoint was investigator-assessed complete response (CR)/unconfirmed CR (CRu) at the end of induction (EOI). Secondary endpoints included safety, survival, treatment satisfaction (Cancer Treatment Satisfaction Questionnaire [CTSQ], Rituximab Administration Satisfaction Questionnaire [RASQ]) and time savings. Follow-up continued until at least 24 months after EOI in the last patient recruited.
Results
Of 576 pts (381 SC; 195 IV), 572 (378 SC; 194 IV) received treatment. EOI CR/CRu rates were 50.6% (95% CI 45.3–55.9) and 42.4 (95% CI 35.1–49.7) in the SC and IV groups, respectively (Table). After 35 months’ median follow-up, median progression-free survival (PFS), event-free survival (EFS) and overall survival (OS) were not reached in either arm and no statistically significant differences were observed between treatment arms. PFS, EFS and OS rates were also similar at 24 months’ follow-up (non-significant differences; Table). Grade ³3 adverse events (58.3% SC; 54.3% IV) and administration-related reactions (21% in both groups) were similar between arms. Of SC recipients, 5.7% had injection site reactions vs none in the IV group (p<0.001). Febrile neutropenia occurred more often in the SC arm (12.5% vs 6.9% in IV, p=0.06). RASQ scores for ‘impact on activities of daily living’, ‘convenience’ and ‘satisfaction’ were improved with SC vs IV; CTSQ scores were similar between arms (Figure). When pts in the SC group were asked, if given the option, which treatment they would prefer, 90.8% stated a preference for SC over IV. Median administration time (6 minutes SC vs 2.6–3.0 hours IV) and chair/bed and overall hospital times were shorter with SC than with IV treatment.
Table. Efficacy endpoints in the intent-to-treat population | ||||
Efficacy endpoint | Rituximab SC plus CHOP | Rituximab IV plus CHOP | ||
N | % (95% CI) | N | % (95% CI) | |
End of induction treatment | ||||
CR/CRu | 342 | 50.6 (45.3–55.9) | 177 | 42.4 (35.1–49.7) |
PR | 342 | 31.6 (26.7–36.8) | 177 | 35.6 (28.6–43.1) |
PD | 342 | 3.8 (2.0–6.4) | 177 | 6.2 (3.1–10.8) |
ORR | 342 | 82.2 (77.7–86.1) | 177 | 78.0 (71.1–83.8) |
24 months’ follow-up | ||||
PFS* | 342 | 67.5 (62.6–72.5) | 177 | 72.3 (65.7–78.9) |
EFS† | 342 | 61.7 (56.5–66.8) | 177 | 66.7 (59.7–73.6) |
OS | 342 | 87.4 (83.2–90.5) | 177 | 88.0 (82.0–92.1) |
*p=0.264. †p=0.265. ORR, overall response rate; PD, progressive disease; PR, partial response. | ||||

Conclusion
Rituximab SC had similar efficacy and safety to the IV form, with improvements in patient satisfaction ratings, and administration/hospital time savings. Our findings support the use of rituximab SC in this setting.
Session topic: 20. Aggressive Non-Hodgkin lymphoma - Clinical
Keyword(s): Diffuse large B cell lymphoma, CHOP, Rituximab


