
Contributions
Abstract: S103
Type: Oral Presentation
Presentation during EHA22: On Friday, June 23, 2017 from 12:15 - 12:30
Location: Hall A
Background
Immunotherapy has emerged as a potentially curative treatment in hematological malignancies. Uniformly expressed in plasma cells, B-cell maturation antigen (BCMA) is an appropriate target antigens for CAR T-cell therapies in multiple myeloma.
Aims
This phase I, open-label trial was conducted to assess the efficacy and safety profile of LCAR-B38M anti-BCMA CAR T cells in patients with relapsed/refractory multiple myeloma.
Methods
All patients underwent leukapheresis to obtain peripheral blood mononuclear cells and their T cells were engineered to express anti-BCMA CAR. Three doses of 300 mg/m2 cyclophosphamide were administered on day -5, -4, and -3 (before the recruitment, patients took the same chemotherapy to identify they were refractory to cyclophosphamide monotherapy) and engineered-T cells were reinfused on day 0, 2, and 6. This trial was divided into the dose escalation stage and expansion cohort. Toxicity and responses were assessed according to the Common Terminology Criteria for Adverse Events (version 4.0) and International Myeloma Working Group (IMWG) Uniform Response Criteria for Multiple Myeloma, respectively.
Results
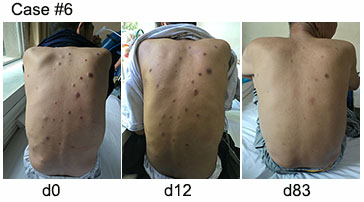
As of the February 20th, 2017 data cut-off, 22 patients had been enrolled, two of whom were diagnosed as plasma cell leukemia. The male: female ratio was 11:11 and median age was 53.5 years. Chromosomal abnormalities were detectable by FISH in eight patients, two of whom involved in the deficiency of p53. Eleven patients were triple refractory (chemotherapy, proteasome inhibitors, and immunomodulatory drugs), 11 resisted to double prior treatments (chemotherapy and proteasome inhibitors/ immunomodulatory drugs), and four relapsed after autologous hematopoietic stem cell transplant. The median number of infused CAR T cells was 4.0×10E6 (range, 1.5×10E6-7.0×10E6) per kg. The median follow-up was 131.5 (range, 29-327) days. 100% of patients achieved an objective response. The first six patients achieved complete responses with flow MRD-negative; 14 patients achieved very good partial responses; one patient, with renal failure, achieved partial response; all these 22 patients had kept their best response at the end of follow-up. The pictures we enclosed were the subcutaneous nodules in one patient with extramedullary plasmacytoma. We found that the nodules were obviously decreased after the infusion and disappeared finally. Another one achieved transient partial response, which last for 12 days. He then took the secondary infusion but failed since the post-operation large-dose administration of corticosteroid for spinal meningioma. He terminally died of the progression of myeloma. The most common toxicity attributable to CAR T cells was cytokine release syndrome (CRS). Toxicities were minimal except for two grade 3 CRS and one grade 4 CRS. All CRSs were controllable with nonsteroidal anti-inflammatory drugs (NSAIDs) or tocilizumab and no dose-limiting toxicities or treatment-related deaths were observed.
Conclusion
Our findings demonstrated the safety and antimyeloma activity of LCAR-B38M anti-BCMA CAR T cells.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Safety, Remission, Multiple Myeloma, Immunotherapy
Abstract: S103
Type: Oral Presentation
Presentation during EHA22: On Friday, June 23, 2017 from 12:15 - 12:30
Location: Hall A
Background
Immunotherapy has emerged as a potentially curative treatment in hematological malignancies. Uniformly expressed in plasma cells, B-cell maturation antigen (BCMA) is an appropriate target antigens for CAR T-cell therapies in multiple myeloma.
Aims
This phase I, open-label trial was conducted to assess the efficacy and safety profile of LCAR-B38M anti-BCMA CAR T cells in patients with relapsed/refractory multiple myeloma.
Methods
All patients underwent leukapheresis to obtain peripheral blood mononuclear cells and their T cells were engineered to express anti-BCMA CAR. Three doses of 300 mg/m2 cyclophosphamide were administered on day -5, -4, and -3 (before the recruitment, patients took the same chemotherapy to identify they were refractory to cyclophosphamide monotherapy) and engineered-T cells were reinfused on day 0, 2, and 6. This trial was divided into the dose escalation stage and expansion cohort. Toxicity and responses were assessed according to the Common Terminology Criteria for Adverse Events (version 4.0) and International Myeloma Working Group (IMWG) Uniform Response Criteria for Multiple Myeloma, respectively.
Results
As of the February 20th, 2017 data cut-off, 22 patients had been enrolled, two of whom were diagnosed as plasma cell leukemia. The male: female ratio was 11:11 and median age was 53.5 years. Chromosomal abnormalities were detectable by FISH in eight patients, two of whom involved in the deficiency of p53. Eleven patients were triple refractory (chemotherapy, proteasome inhibitors, and immunomodulatory drugs), 11 resisted to double prior treatments (chemotherapy and proteasome inhibitors/ immunomodulatory drugs), and four relapsed after autologous hematopoietic stem cell transplant. The median number of infused CAR T cells was 4.0×10E6 (range, 1.5×10E6-7.0×10E6) per kg. The median follow-up was 131.5 (range, 29-327) days. 100% of patients achieved an objective response. The first six patients achieved complete responses with flow MRD-negative; 14 patients achieved very good partial responses; one patient, with renal failure, achieved partial response; all these 22 patients had kept their best response at the end of follow-up. The pictures we enclosed were the subcutaneous nodules in one patient with extramedullary plasmacytoma. We found that the nodules were obviously decreased after the infusion and disappeared finally. Another one achieved transient partial response, which last for 12 days. He then took the secondary infusion but failed since the post-operation large-dose administration of corticosteroid for spinal meningioma. He terminally died of the progression of myeloma. The most common toxicity attributable to CAR T cells was cytokine release syndrome (CRS). Toxicities were minimal except for two grade 3 CRS and one grade 4 CRS. All CRSs were controllable with nonsteroidal anti-inflammatory drugs (NSAIDs) or tocilizumab and no dose-limiting toxicities or treatment-related deaths were observed.

Conclusion
Our findings demonstrated the safety and antimyeloma activity of LCAR-B38M anti-BCMA CAR T cells.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Safety, Remission, Multiple Myeloma, Immunotherapy


