
Contributions
Abstract: S101
Type: Oral Presentation
Presentation during EHA22: On Friday, June 23, 2017 from 11:45 - 12:00
Location: Hall A
Background
Daratumumab (D) is a human CD38-targeting monoclonal antibody that exerts its antimyeloma activity through both direct (on-tumor) and indirect (immunomodulatory) mechanisms of action. Two randomized phase 3 trials in patients with relapsed or refractory multiple myeloma (RRMM) demonstrated that combining D with the standard-of-care regimens lenalidomide + dexamethasone (Rd, POLLUX) or bortezomib + dexamethasone (Vd, CASTOR) significantly improved progression-free survival (PFS) and achieved higher overall response rates (ORRs) compared with the respective standard-of-care regimen alone (Dimopoulos MA et al, N Engl J Med 2016;375(14):1319-1331; Palumbo A et al, N Engl J Med 2016;375(8):754-766.). Due to its novel mechanisms of action, addition of D to standard-of-care regimens may benefit RRMM patients who have poor prognoses resulting from high-risk cytogenetic abnormalities.
Aims
To examine the efficacy of DRd and DVd in RRMM patients with standard or high cytogenetic risk status.
Methods
Bone marrow aspirates were collected at screening visits from 311/569 patients from POLLUX and from 353/498 patients from CASTOR, and cytogenetic abnormalities were detected via next-generation sequencing (NGS). Patients were considered to be of high cytogenetic risk status if they had ≥1 of the following abnormalities: t(4;14), t(14;16), or del17p; patients were considered to be of standard cytogenetic risk if they lacked these abnormalities. Minimal residual disease (MRD) was assessed at suspected complete response (CR) at 3 sensitivity thresholds (10–4, 10–5, and 10–6) using the ClonoSEQ™ NGS-based assay (Adaptive Biotechnologies, Seattle, WA). Efficacy analyses included PFS, ORR, and MRD-negative rates.
Results
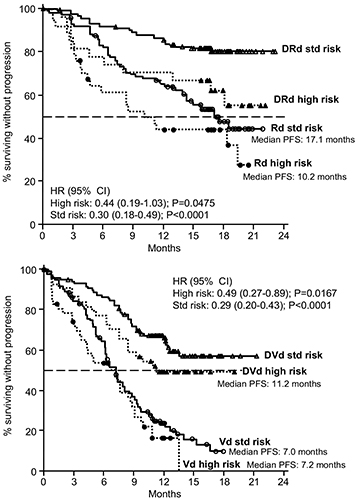
Conclusion
Adding D to Rd or Vd improved treatment outcomes irrespective of cytogenetic risk status in patients with RRMM. Both DRd and DVd appear to benefit RRMM patients who have poor prognoses due to high-risk cytogenetic abnormalities. Updated data, including analyses based on individual cytogenetic abnormalities, will be presented at the meeting based on longer follow-up.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): CD38, Multiple Myeloma, Minimal residual disease (MRD), Cytogenetic abnormalities
Abstract: S101
Type: Oral Presentation
Presentation during EHA22: On Friday, June 23, 2017 from 11:45 - 12:00
Location: Hall A
Background
Daratumumab (D) is a human CD38-targeting monoclonal antibody that exerts its antimyeloma activity through both direct (on-tumor) and indirect (immunomodulatory) mechanisms of action. Two randomized phase 3 trials in patients with relapsed or refractory multiple myeloma (RRMM) demonstrated that combining D with the standard-of-care regimens lenalidomide + dexamethasone (Rd, POLLUX) or bortezomib + dexamethasone (Vd, CASTOR) significantly improved progression-free survival (PFS) and achieved higher overall response rates (ORRs) compared with the respective standard-of-care regimen alone (Dimopoulos MA et al, N Engl J Med 2016;375(14):1319-1331; Palumbo A et al, N Engl J Med 2016;375(8):754-766.). Due to its novel mechanisms of action, addition of D to standard-of-care regimens may benefit RRMM patients who have poor prognoses resulting from high-risk cytogenetic abnormalities.
Aims
To examine the efficacy of DRd and DVd in RRMM patients with standard or high cytogenetic risk status.
Methods
Bone marrow aspirates were collected at screening visits from 311/569 patients from POLLUX and from 353/498 patients from CASTOR, and cytogenetic abnormalities were detected via next-generation sequencing (NGS). Patients were considered to be of high cytogenetic risk status if they had ≥1 of the following abnormalities: t(4;14), t(14;16), or del17p; patients were considered to be of standard cytogenetic risk if they lacked these abnormalities. Minimal residual disease (MRD) was assessed at suspected complete response (CR) at 3 sensitivity thresholds (10–4, 10–5, and 10–6) using the ClonoSEQ™ NGS-based assay (Adaptive Biotechnologies, Seattle, WA). Efficacy analyses included PFS, ORR, and MRD-negative rates.
Results

Conclusion
Adding D to Rd or Vd improved treatment outcomes irrespective of cytogenetic risk status in patients with RRMM. Both DRd and DVd appear to benefit RRMM patients who have poor prognoses due to high-risk cytogenetic abnormalities. Updated data, including analyses based on individual cytogenetic abnormalities, will be presented at the meeting based on longer follow-up.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): CD38, Multiple Myeloma, Minimal residual disease (MRD), Cytogenetic abnormalities


