
Contributions
Abstract: S100
Type: Oral Presentation
Presentation during EHA22: On Friday, June 23, 2017 from 11:30 - 11:45
Location: Hall A
Background
Cytogenetic risk status in multiple myeloma (MM) studies is traditionally determined by using fluorescence in situ hybridization (FISH) or karyotyping to assess chromosomal abnormalities. However, these technologies have limited resolution and a narrow target range, and reproducible interpretation may be confounded by inter-laboratory variation.
Aims
To describe the NGS methodology used to determine cytogenetic risk status in the daratumumab phase 3 CASTOR and POLLUX studies in RRMM.
Methods
Bone marrow aspirates were collected at screening and assessed centrally via NGS. Whole exome sequencing (exome-seq) and RNA sequencing (RNA-seq) was performed using the Illumina HiSeq platform to identify the presence or absence of defined risk markers: t(4;14), t(14;16), or del17p. The use of RNA-seq allowed for investigation of chromosomal translocations in expressed genomic locations at a higher resolution than FISH, and exome-seq data was used to derive the copy number status in coding regions across the genome. RNA-seq was performed using total RNA and rRNA removal to capture translocations involving coding and intronic regions. Translocation calls were made using two fusion callers, and gene expression was quantified to allow for evaluation of genes associated with translocation events. For t(4;14) translocations, the detected events involved RNA-seq reads fused between IgH and WHSC1 or FGFR3. For t(14;16), the detected translocations involved IgH and WWOX. Manual inspection of patients with t(4;14) showed higher WHSC1 or FGFR3 expression, whereas t(14;16) patients showed higher MAF and CCND2 expression. For del17p detection, exome data of each tumor was compared against 100 peripheral blood mononuclear cell (PBMC) control samples from CASTOR and POLLUX studies. Copy number variation data from two callers were combined to utilize information on relative read depth, systematic biases (observed in pooled normal controls), as well as SNP allele frequency (indicative of loss of heterozygosity events). A del17p event was detected when >50% of the 17p region was deleted.
Results
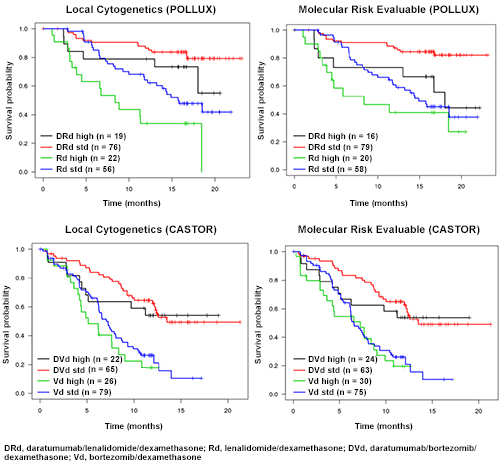
Based on the RNA-Seq and exome results, cytogenetic risk status in the CASTOR and POLLUX studies was defined as high risk with having either t(4;14), t(14;16), or del17p, and standard risk with the confirmed absence of these molecular abnormalities. Comparisons of NGS with FISH showed high concordance for t(4;14), t(14;16), and del17p in both studies (Table). PFS analyses investigating differences between treatment groups and between risk groups using FISH-derived risk and NGS-derived risk showed consistent results between FISH and NGS, with improvements in PFS being associated with the addition of daratumumab to standard-of-care regimens in both high- and standard-risk subgroups (Figure).
Concordance rate between FISH and NGS | POLLUX | CASTOR |
t(4;14) | 96% | 92% |
t(14;16) | 98% | 97% |
del17p | 88% | 90% |
Conclusion
These studies represent the first, comprehensive use of NGS in global phase 3 clinical trials in RRMM. The NGS methodology accurately identified the presence of defined risk populations t(4;14), t(14;16), and del17p and showed good concordance with FISH. As FISH was performed locally with different probes and pathologists, the high degree of concordance between FISH and NGS is notable and supports the use of NGS for determining cytogenetic risk in patients with RRMM. The utility of NGS in these clinical studies extends far beyond the detection of cytogenetic abnormalities and additional analysis are planned to interrogate these datasets in the identification of novel biomarkers.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Multiple Myeloma, Immunotherapy, Cytogenetic abnormalities, CD38
Abstract: S100
Type: Oral Presentation
Presentation during EHA22: On Friday, June 23, 2017 from 11:30 - 11:45
Location: Hall A
Background
Cytogenetic risk status in multiple myeloma (MM) studies is traditionally determined by using fluorescence in situ hybridization (FISH) or karyotyping to assess chromosomal abnormalities. However, these technologies have limited resolution and a narrow target range, and reproducible interpretation may be confounded by inter-laboratory variation.
Aims
To describe the NGS methodology used to determine cytogenetic risk status in the daratumumab phase 3 CASTOR and POLLUX studies in RRMM.
Methods
Bone marrow aspirates were collected at screening and assessed centrally via NGS. Whole exome sequencing (exome-seq) and RNA sequencing (RNA-seq) was performed using the Illumina HiSeq platform to identify the presence or absence of defined risk markers: t(4;14), t(14;16), or del17p. The use of RNA-seq allowed for investigation of chromosomal translocations in expressed genomic locations at a higher resolution than FISH, and exome-seq data was used to derive the copy number status in coding regions across the genome. RNA-seq was performed using total RNA and rRNA removal to capture translocations involving coding and intronic regions. Translocation calls were made using two fusion callers, and gene expression was quantified to allow for evaluation of genes associated with translocation events. For t(4;14) translocations, the detected events involved RNA-seq reads fused between IgH and WHSC1 or FGFR3. For t(14;16), the detected translocations involved IgH and WWOX. Manual inspection of patients with t(4;14) showed higher WHSC1 or FGFR3 expression, whereas t(14;16) patients showed higher MAF and CCND2 expression. For del17p detection, exome data of each tumor was compared against 100 peripheral blood mononuclear cell (PBMC) control samples from CASTOR and POLLUX studies. Copy number variation data from two callers were combined to utilize information on relative read depth, systematic biases (observed in pooled normal controls), as well as SNP allele frequency (indicative of loss of heterozygosity events). A del17p event was detected when >50% of the 17p region was deleted.
Results
Based on the RNA-Seq and exome results, cytogenetic risk status in the CASTOR and POLLUX studies was defined as high risk with having either t(4;14), t(14;16), or del17p, and standard risk with the confirmed absence of these molecular abnormalities. Comparisons of NGS with FISH showed high concordance for t(4;14), t(14;16), and del17p in both studies (Table). PFS analyses investigating differences between treatment groups and between risk groups using FISH-derived risk and NGS-derived risk showed consistent results between FISH and NGS, with improvements in PFS being associated with the addition of daratumumab to standard-of-care regimens in both high- and standard-risk subgroups (Figure).
Concordance rate between FISH and NGS | POLLUX | CASTOR |
t(4;14) | 96% | 92% |
t(14;16) | 98% | 97% |
del17p | 88% | 90% |

Conclusion
These studies represent the first, comprehensive use of NGS in global phase 3 clinical trials in RRMM. The NGS methodology accurately identified the presence of defined risk populations t(4;14), t(14;16), and del17p and showed good concordance with FISH. As FISH was performed locally with different probes and pathologists, the high degree of concordance between FISH and NGS is notable and supports the use of NGS for determining cytogenetic risk in patients with RRMM. The utility of NGS in these clinical studies extends far beyond the detection of cytogenetic abnormalities and additional analysis are planned to interrogate these datasets in the identification of novel biomarkers.
Session topic: 14. Myeloma and other monoclonal gammopathies - Clinical
Keyword(s): Multiple Myeloma, Immunotherapy, Cytogenetic abnormalities, CD38


