
Contributions
Abstract: O08
Type: Oral presentation
Session Topic: Inherited and acquired disorders of platelets
Presentation during EHA Scientific Conference on Bleeding Disorders:
On Thursday, September 15, 2016 from 09:00 - 10:30
Location: Rossini 1
Introduction
Quantitative and/or qualitative defects of the platelet membrane glycoprotein (GP) IIb/IIIa complex lead to the clinical entity of Glanzmann's thrombasthenia (GT). A large variety of mutations and polymorphisms are responsible for the aberrant expression and defective activity of this heterodimeric complex. The present study aimed to determine the pattern of GT mutations in Iranian population with GT.
Materials and methods
We evaluated 20 patients with GT. All exons and splice sites of ITGA2B and ITGB3 were amplified by Touchdown PCR. Mutation screening were analyzed by CSGE heteroduplex PCR and DNA sequencing. Immunophenotypic analysis was performed by flow cytometry.
Results
We identified three novel mutations, one previously identified mutation and three polymorphisms which two of them were novel. in detailed, one substitution mutation, two deletions of a single nucleotide, one insertion of a single nucleotide, two synonymous polymorphisms and one missense polymorphism were found.
Discussion
All detected mutations were homozygous which likely contribute to the pathogenesis of GT. Furthermore, it suggested ITGB3 as the mainly affected glycoprotein impaired in the patients with GT. As expected, the molecular results were consistent with the phenotypic findings, so that the GPIIb/IIIa complex was disrupted by mutations in all studied patients with type I GT. Finally, we concluded that intronic alterations or epigenetic regulation is responsible for the aberrant expression and/or defective activity of GPIIb/IIIa complex in the other patients.
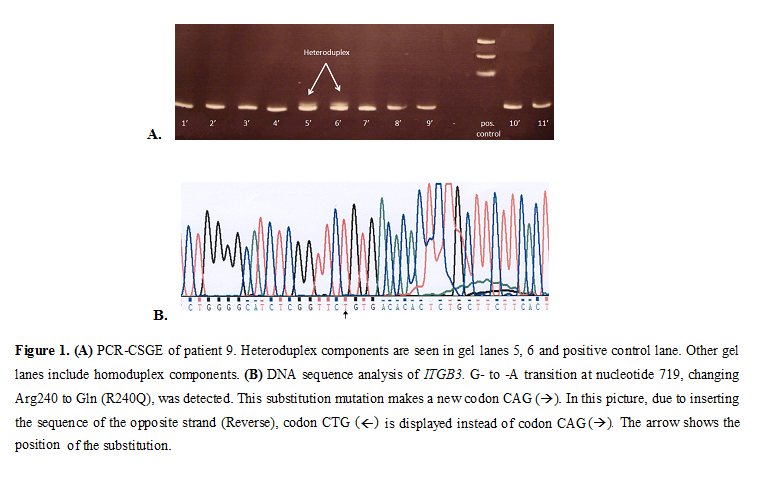
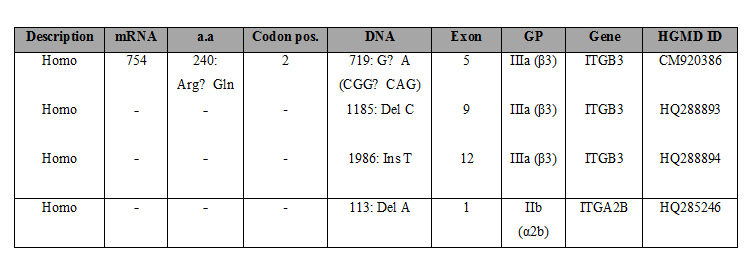
Keywords
Glanzmann's Thrombasthenia, ITGA2B, ITGB3, GPIIb/IIIa complex, Novel mutations and polymorphisms
References
1. Farsinejad A, Farajollahi MM, Kazemi A, Saemi N, Faranoush M. Different biochemical expression pattern of platelet surface glycoproteins suggests molecular diversity of Glanzmann's thrombasthenia in Iran. Blood Coagulation & Fibrinolysis. 2013;24(6):613-8.
2. Mansour W, Einav Y, Hauschner H, Koren A, Seligsohn U, Rosenberg N. An αIIb mutation in patients with Glanzmann thrombasthenia located in the N‐terminus of blade 1 of the β‐propeller (Asn2Asp) disrupts a calcium binding site in blade 6. Journal of Thrombosis and Haemostasis. 2011;9(1):192-200.
3. Jallu V, Dusseaux M, Panzer S, Torchet MF, Hezard N, Goudemand J, et al. αIIbβ3 Integrin: new allelic variants in Glanzmann thrombasthenia, effects on ITGA2B and ITGB3 mRNA splicing, expression, and structure–function. Human mutation. 2010;31(3):237-46.
4. Vannier C, Behnisch W, Bartsch I, Sandrock K, Ertle F, Schmidt K, et al. Novel homozygous mutation (c. 175delG) in platelet glycoprotein ITGA2B gene as cause of Glanzmann's thrombasthenia type I. Klinische Padiatrie. 2010;222(3):150-3.
5. Sandrock K, Halimeh S, Wiegering V, Kappert G, Sauer K, Deeg N, et al. Homozygous Point Mutations in Platelet Glycoprotein ITGA2B Gene as Cause of Glanzmann Thrombasthenia in 2 Families. Klinische Pädiatrie. 2012;224(3):174.
6. Nurden AT, Fiore M, Nurden P, Pillois X. Glanzmann thrombasthenia: a review of ITGA2B and ITGB3 defects with emphasis on variants, phenotypic variability, and mouse models. Blood. 2011;118(23):5996-6005.
7. Nurden AT, Pillois X, Nurden P. Understanding the genetic basis of Glanzmann thrombasthenia: implications for treatment. Expert review of hematology. 2012;5(5):487-503.
8. Haghighi A, Borhany M, Ghazi A, Edwards N, Tabaksert A, Fatima N, et al. Glanzmann thrombasthenia in Pakistan: molecular analysis and identification of novel mutations. Clinical genetics. 2016;89(2):187-92.
9. Peretz H, Rosenberg N, Landau M, Usher S, Nelson EJ, Mor‐Cohen R, et al. Molecular diversity of Glanzmann thrombasthenia in southern India: new insights into mRNA splicing and structure–function correlations of αIIbβ3 integrin (ITGA2B, ITGB3). Human mutation. 2006;27(4):359-69.
10. Pillitteri D, Pilgrimm A-K, Kirchmaier CM. Novel mutations in the GPIIb and GPIIIa genes in glanzmann thrombasthenia. Transfusion Medicine and Hemotherapy. 2010;37(5):268-77.
11. Xu X, Liu Y, Ying Y, Tao S, Hong X, Zhu F, et al. Human platelet antigen allele frequencies and new mutations on platelet glycoprotein genes in the Chinese Han population. Transfusion Medicine. 2011;21(5):330-7.
12. Rosenberg N, Hauschner H, Peretz H, MOR‐COHEN R, Landau M, Shenkman B, et al. A 13‐bp deletion in αIIb gene is a founder mutation that predominates in Palestinian‐Arab patients with Glanzmann thrombasthenia. Journal of Thrombosis and Haemostasis. 2005;3(12):2764-72.
13. Feng X, Novack DV, Faccio R, Ory DS, Aya K, Boyer MI, et al. A Glanzmann’s mutation in β3 integrin specifically impairs osteoclast function. The Journal of clinical investigation. 2001;107(9):1137-44.
14. Coller BS, Seligsohn U, Peretz H, Newman PJ, editors. Glanzmann thrombasthenia: new insights from an historical perspective. Seminars in hematology; 1994.
15. Nair S, Li J, Mitchell WB, Mohanty D, Coller BS, French DL. Two New β3 Integrin Mutations in Indian Patients with Glanzmann Thrombasthenia: Localization of Mutations affecting Cysteine Residues in Integrin β3. Thromb Haemost. 2002;88(3):503-9.
16. Jacquelin B, Tuleja E, Kunicki T, Nurden P, Nurden A. Analysis of platelet membrane glycoprotein polymorphisms in Glanzmann thrombasthenia showed the French gypsy mutation in the αIIb gene to be strongly linked to the HPA‐1b polymorphism in β3. Journal of Thrombosis and Haemostasis. 2003;1(3):573-5.
17. Nurden AT, Pillois X, Fiore M, Alessi MC, Bonduel M, Dreyfus M, et al. Expanding the Mutation Spectrum Affecting αIIbβ3 Integrin in Glanzmann Thrombasthenia: Screening of the ITGA2B and ITGB3 Genes in a Large International Cohort. Human mutation. 2015;36(5):548-61.
18. Coller B. αIIbβ3: structure and function. Journal of Thrombosis and Haemostasis. 2015;13(S1):S17-S25.
19. Buitrago L, Rendon A, Liang Y, Simeoni I, Negri A, Filizola M, et al. αIIbβ3 variants defined by next-generation sequencing: Predicting variants likely to cause Glanzmann thrombasthenia. Proceedings of the National Academy of Sciences. 2015;112(15):E1898-E907.
20. Tokgoz H, Torun Ozkan D, Caliskan U, Akar N. Novel mutations of integrin αIIb and β3 genes in Turkish children with Glanzmann’s thrombasthenia. Platelets. 2015;26(8):779-82.
21. Pillois X, Fiore M, Heilig R, Pico M, Nurden AT. A novel amino acid substitution of integrin αIIb in Glanzmann thrombasthenia confirms that the N-terminal region of the receptor plays a role in maintaining β-propeller structure. Platelets. 2013;24(1):77-80.
22. Ruiz C, Liu C-Y, Sun Q-H, Sigaud-Fiks M, Fressinaud E, Muller J-Y, et al. A point mutation in the cysteine-rich domain of glycoprotein (GP) IIIa results in the expression of a GPIIb-IIIa (αIIbβ3) integrin receptor locked in a high-affinity state and a Glanzmann thrombasthenia–like phenotype. Blood. 2001;98(8):2432-41.
23. Rosenberg N, Yatuv R, Sobolev V, Peretz H, Zivelin A, Seligsohn U. Major mutations in calf-1 and calf-2 domains of glycoprotein IIb in patients with Glanzmann thrombasthenia enable GPIIb/IIIa complex formation, but impair its transport from the endoplasmic reticulum to the Golgi apparatus. Blood. 2003;101(12):4808-15.
24. Peretz H, Rosenberg N, Usher S, Graff E, Newman P, Coller BS, et al. Glanzmann's thrombasthenia associated with deletion-insertion and alternative splicing in the glycoprotein IIb gene. Blood. 1995;85(2):414-20.
25. Franchini M, Favaloro EJ, Lippi G. Glanzmann thrombasthenia: an update. Clinica Chimica Acta. 2010;411(1):1-6.
26. Sauna ZE, Kimchi-Sarfaty C, Ambudkar SV, Gottesman MM. Silent polymorphisms speak: how they affect pharmacogenomics and the treatment of cancer. Cancer Research. 2007;67(20):9609-12.
27. Park S, Park H, Park C. Association of the gene polymorphism of platelet glycoprotein Ia and IIb/IIIa with myocardial infarction and extent of coronary artery disease in the Korean population. Yonsei Med J. 2004;45:428-34.
28. Khatami M, Heidari MM. Common rs5918 (PlA1/A2) polymorphism in the ITGB3 gene and risk of coronary artery disease. 2016.
29. Xiang Q, Ji S-D, Zhang Z, Zhao X, Cui Y-M. Identification of ITGA2B and ITGB3 single-nucleotide polymorphisms and their influences on the platelet function.
30. Ghosh K, Kulkarni B, Nair S, Shetty S, Mohanty D. Human platelet alloantigen polymorphism in Glanzmann's thrombasthenia and its impact on the severity of the disease. British journal of haematology. 2002;119(2):348-53.
Abstract: O08
Type: Oral presentation
Session Topic: Inherited and acquired disorders of platelets
Presentation during EHA Scientific Conference on Bleeding Disorders:
On Thursday, September 15, 2016 from 09:00 - 10:30
Location: Rossini 1
Introduction
Quantitative and/or qualitative defects of the platelet membrane glycoprotein (GP) IIb/IIIa complex lead to the clinical entity of Glanzmann's thrombasthenia (GT). A large variety of mutations and polymorphisms are responsible for the aberrant expression and defective activity of this heterodimeric complex. The present study aimed to determine the pattern of GT mutations in Iranian population with GT.
Materials and methods
We evaluated 20 patients with GT. All exons and splice sites of ITGA2B and ITGB3 were amplified by Touchdown PCR. Mutation screening were analyzed by CSGE heteroduplex PCR and DNA sequencing. Immunophenotypic analysis was performed by flow cytometry.
Results
We identified three novel mutations, one previously identified mutation and three polymorphisms which two of them were novel. in detailed, one substitution mutation, two deletions of a single nucleotide, one insertion of a single nucleotide, two synonymous polymorphisms and one missense polymorphism were found.
Discussion
All detected mutations were homozygous which likely contribute to the pathogenesis of GT. Furthermore, it suggested ITGB3 as the mainly affected glycoprotein impaired in the patients with GT. As expected, the molecular results were consistent with the phenotypic findings, so that the GPIIb/IIIa complex was disrupted by mutations in all studied patients with type I GT. Finally, we concluded that intronic alterations or epigenetic regulation is responsible for the aberrant expression and/or defective activity of GPIIb/IIIa complex in the other patients.


Keywords
Glanzmann's Thrombasthenia, ITGA2B, ITGB3, GPIIb/IIIa complex, Novel mutations and polymorphisms
References
1. Farsinejad A, Farajollahi MM, Kazemi A, Saemi N, Faranoush M. Different biochemical expression pattern of platelet surface glycoproteins suggests molecular diversity of Glanzmann's thrombasthenia in Iran. Blood Coagulation & Fibrinolysis. 2013;24(6):613-8.
2. Mansour W, Einav Y, Hauschner H, Koren A, Seligsohn U, Rosenberg N. An αIIb mutation in patients with Glanzmann thrombasthenia located in the N‐terminus of blade 1 of the β‐propeller (Asn2Asp) disrupts a calcium binding site in blade 6. Journal of Thrombosis and Haemostasis. 2011;9(1):192-200.
3. Jallu V, Dusseaux M, Panzer S, Torchet MF, Hezard N, Goudemand J, et al. αIIbβ3 Integrin: new allelic variants in Glanzmann thrombasthenia, effects on ITGA2B and ITGB3 mRNA splicing, expression, and structure–function. Human mutation. 2010;31(3):237-46.
4. Vannier C, Behnisch W, Bartsch I, Sandrock K, Ertle F, Schmidt K, et al. Novel homozygous mutation (c. 175delG) in platelet glycoprotein ITGA2B gene as cause of Glanzmann's thrombasthenia type I. Klinische Padiatrie. 2010;222(3):150-3.
5. Sandrock K, Halimeh S, Wiegering V, Kappert G, Sauer K, Deeg N, et al. Homozygous Point Mutations in Platelet Glycoprotein ITGA2B Gene as Cause of Glanzmann Thrombasthenia in 2 Families. Klinische Pädiatrie. 2012;224(3):174.
6. Nurden AT, Fiore M, Nurden P, Pillois X. Glanzmann thrombasthenia: a review of ITGA2B and ITGB3 defects with emphasis on variants, phenotypic variability, and mouse models. Blood. 2011;118(23):5996-6005.
7. Nurden AT, Pillois X, Nurden P. Understanding the genetic basis of Glanzmann thrombasthenia: implications for treatment. Expert review of hematology. 2012;5(5):487-503.
8. Haghighi A, Borhany M, Ghazi A, Edwards N, Tabaksert A, Fatima N, et al. Glanzmann thrombasthenia in Pakistan: molecular analysis and identification of novel mutations. Clinical genetics. 2016;89(2):187-92.
9. Peretz H, Rosenberg N, Landau M, Usher S, Nelson EJ, Mor‐Cohen R, et al. Molecular diversity of Glanzmann thrombasthenia in southern India: new insights into mRNA splicing and structure–function correlations of αIIbβ3 integrin (ITGA2B, ITGB3). Human mutation. 2006;27(4):359-69.
10. Pillitteri D, Pilgrimm A-K, Kirchmaier CM. Novel mutations in the GPIIb and GPIIIa genes in glanzmann thrombasthenia. Transfusion Medicine and Hemotherapy. 2010;37(5):268-77.
11. Xu X, Liu Y, Ying Y, Tao S, Hong X, Zhu F, et al. Human platelet antigen allele frequencies and new mutations on platelet glycoprotein genes in the Chinese Han population. Transfusion Medicine. 2011;21(5):330-7.
12. Rosenberg N, Hauschner H, Peretz H, MOR‐COHEN R, Landau M, Shenkman B, et al. A 13‐bp deletion in αIIb gene is a founder mutation that predominates in Palestinian‐Arab patients with Glanzmann thrombasthenia. Journal of Thrombosis and Haemostasis. 2005;3(12):2764-72.
13. Feng X, Novack DV, Faccio R, Ory DS, Aya K, Boyer MI, et al. A Glanzmann’s mutation in β3 integrin specifically impairs osteoclast function. The Journal of clinical investigation. 2001;107(9):1137-44.
14. Coller BS, Seligsohn U, Peretz H, Newman PJ, editors. Glanzmann thrombasthenia: new insights from an historical perspective. Seminars in hematology; 1994.
15. Nair S, Li J, Mitchell WB, Mohanty D, Coller BS, French DL. Two New β3 Integrin Mutations in Indian Patients with Glanzmann Thrombasthenia: Localization of Mutations affecting Cysteine Residues in Integrin β3. Thromb Haemost. 2002;88(3):503-9.
16. Jacquelin B, Tuleja E, Kunicki T, Nurden P, Nurden A. Analysis of platelet membrane glycoprotein polymorphisms in Glanzmann thrombasthenia showed the French gypsy mutation in the αIIb gene to be strongly linked to the HPA‐1b polymorphism in β3. Journal of Thrombosis and Haemostasis. 2003;1(3):573-5.
17. Nurden AT, Pillois X, Fiore M, Alessi MC, Bonduel M, Dreyfus M, et al. Expanding the Mutation Spectrum Affecting αIIbβ3 Integrin in Glanzmann Thrombasthenia: Screening of the ITGA2B and ITGB3 Genes in a Large International Cohort. Human mutation. 2015;36(5):548-61.
18. Coller B. αIIbβ3: structure and function. Journal of Thrombosis and Haemostasis. 2015;13(S1):S17-S25.
19. Buitrago L, Rendon A, Liang Y, Simeoni I, Negri A, Filizola M, et al. αIIbβ3 variants defined by next-generation sequencing: Predicting variants likely to cause Glanzmann thrombasthenia. Proceedings of the National Academy of Sciences. 2015;112(15):E1898-E907.
20. Tokgoz H, Torun Ozkan D, Caliskan U, Akar N. Novel mutations of integrin αIIb and β3 genes in Turkish children with Glanzmann’s thrombasthenia. Platelets. 2015;26(8):779-82.
21. Pillois X, Fiore M, Heilig R, Pico M, Nurden AT. A novel amino acid substitution of integrin αIIb in Glanzmann thrombasthenia confirms that the N-terminal region of the receptor plays a role in maintaining β-propeller structure. Platelets. 2013;24(1):77-80.
22. Ruiz C, Liu C-Y, Sun Q-H, Sigaud-Fiks M, Fressinaud E, Muller J-Y, et al. A point mutation in the cysteine-rich domain of glycoprotein (GP) IIIa results in the expression of a GPIIb-IIIa (αIIbβ3) integrin receptor locked in a high-affinity state and a Glanzmann thrombasthenia–like phenotype. Blood. 2001;98(8):2432-41.
23. Rosenberg N, Yatuv R, Sobolev V, Peretz H, Zivelin A, Seligsohn U. Major mutations in calf-1 and calf-2 domains of glycoprotein IIb in patients with Glanzmann thrombasthenia enable GPIIb/IIIa complex formation, but impair its transport from the endoplasmic reticulum to the Golgi apparatus. Blood. 2003;101(12):4808-15.
24. Peretz H, Rosenberg N, Usher S, Graff E, Newman P, Coller BS, et al. Glanzmann's thrombasthenia associated with deletion-insertion and alternative splicing in the glycoprotein IIb gene. Blood. 1995;85(2):414-20.
25. Franchini M, Favaloro EJ, Lippi G. Glanzmann thrombasthenia: an update. Clinica Chimica Acta. 2010;411(1):1-6.
26. Sauna ZE, Kimchi-Sarfaty C, Ambudkar SV, Gottesman MM. Silent polymorphisms speak: how they affect pharmacogenomics and the treatment of cancer. Cancer Research. 2007;67(20):9609-12.
27. Park S, Park H, Park C. Association of the gene polymorphism of platelet glycoprotein Ia and IIb/IIIa with myocardial infarction and extent of coronary artery disease in the Korean population. Yonsei Med J. 2004;45:428-34.
28. Khatami M, Heidari MM. Common rs5918 (PlA1/A2) polymorphism in the ITGB3 gene and risk of coronary artery disease. 2016.
29. Xiang Q, Ji S-D, Zhang Z, Zhao X, Cui Y-M. Identification of ITGA2B and ITGB3 single-nucleotide polymorphisms and their influences on the platelet function.
30. Ghosh K, Kulkarni B, Nair S, Shetty S, Mohanty D. Human platelet alloantigen polymorphism in Glanzmann's thrombasthenia and its impact on the severity of the disease. British journal of haematology. 2002;119(2):348-53.


