TELOMERE LENGTH SHORTENING IS ASSOCIATED WITH TREATMENT-FREE REMISSION IN CHRONIC MYELOID LEUKEMIA PATIENTS
(Abstract release date: 05/19/16)
EHA Library. Caocci G. 06/09/16; 135366; LB2255
Disclosure(s): none

Dr. Giovanni Caocci
Contributions
Contributions
Abstract
Abstract: LB2255
Type: Eposter Presentation
Background
Telomere biology has been more extensively studied in chronic myeloid leukemia (CML) than in any other blood cancer. Shorter telomeres have been associated to disease progression, poor prognosis, higher Hasford score and acquisition of further cytogenetic aberrations. So far, there are no studies investigating the possible influence of telomeres on treatment-free remission (TFR) after discontinuation of therapy with tyrosine kinase inhibitors (TKIs).
Aims
The aim of the present study was to investigate whether telomere length was associated with durable TFR in a cohort of CML patients after discontinuation of treatment with imatinib.
Methods
Thirty-two chronic-phase CML patients discontinued TKI treatment after achieving complete molecular remission (MR4). All patients were treated with imatinib. Two patients underwent second-line TKI (nilotinib) following molecular relapse. Telomere length analysis was performed as described by Cawthon (2002) using a quantitative PCR (q-PCR). The Relative Telomere Length (RTL) was determined as the Telomere (T) to Single copy gene (36B4) (S) ratio (T/S) normalized to a reference sample (K-562 DNA). Peripheral blood samples were also collected from 32 age- and sex-matched healthy controls. Age corrected RTL (acRTL) represented the difference in telomere length between patients and age- and sex-matched controls.
Results
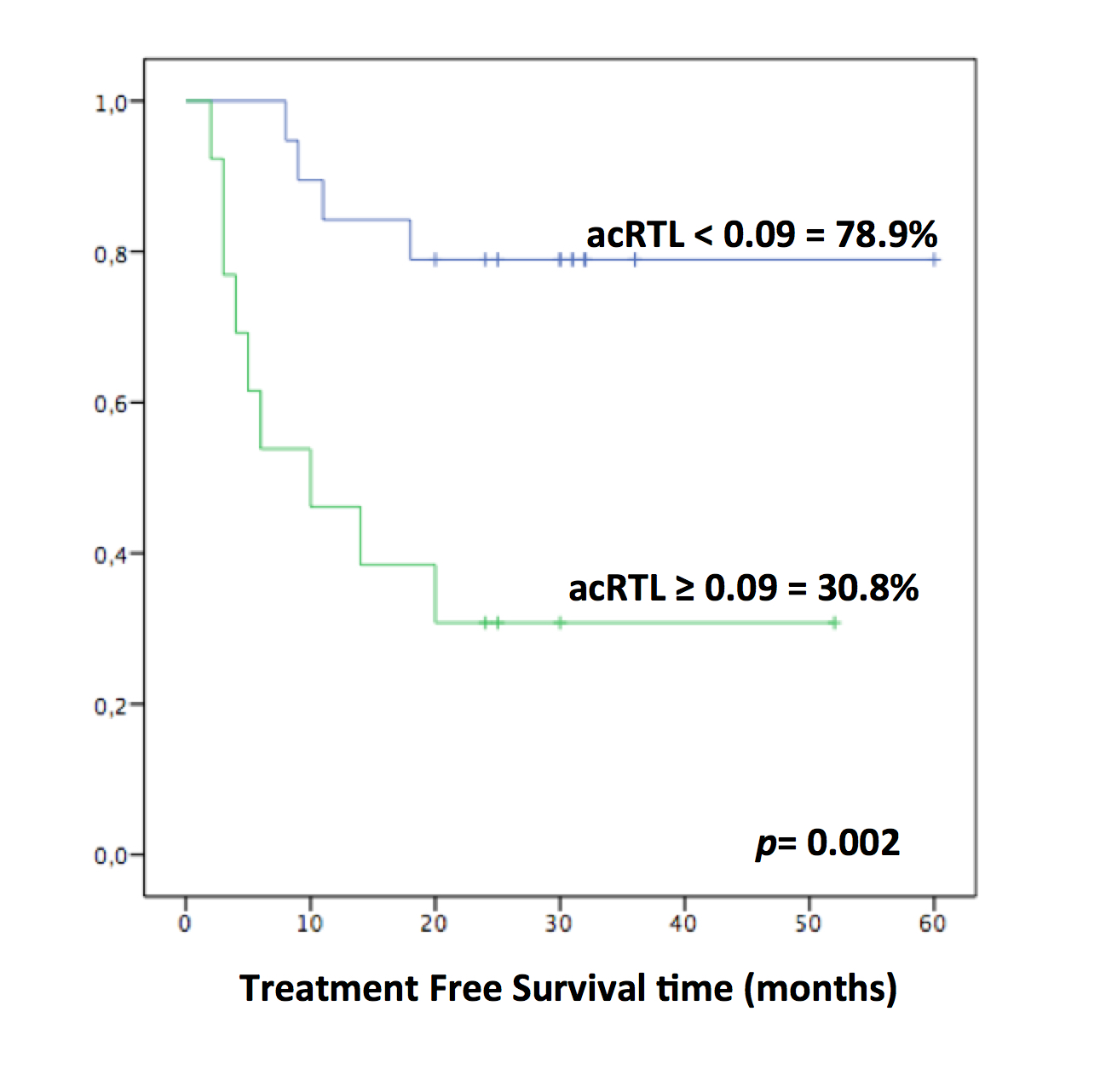
Complete molecular remission (CMR) was achieved after a mean of 26 months of imatinib (range 3-93). TKI treatment was discontinued after a mean of 84 months from the start of treatment (range 24-143). The median follow-up after discontinuation was 30 months (range 18-60). Thirteen patients (41%) showed loss of CMR. All relapsed patients regained CMR after restarting treatment with TKIs. The 36-month cumulative probability of TFR was 59.4%. RTL was assessed at a mean of 32 months from discontinuation in TFR patients and at a mean of 23 months after relapse in others. The median value of acRTL in the CML cohort was 0.09 (range -0.26, +0.86). The Mann-Whitney U test showed shorter acRTL in TFR patients compared to patients with molecular relapse (mean±SD = 0.01±0.14 vs 0.20±0.21; p=0.01). Patients were stratified according to the median value of acRTL ≤0.09. TFR was significantly higher in CML patients with acRTL ≤0.09 in comparison to those with longer telomeres (78.9% vs 30.8%, p=0.002) (Figure).
Conclusion
Achievement of biological recovery through control or eradication of CML stem cells might depend on their proliferative potential in relation to telomere length. Age (a parameter directly proportional to telomere shortening and senescence) has recently been reported as a predictive factor for molecular relapse in CML patients who discontinue imatinib. CML cells would escape senescence through up-regulation of telomerase, restoring telomere length. In our study cohort, TFR patients showed significantly shorter acRTL than patients who presented molecular relapse. It can be postulated that quiescent CML stem cells harboring longer telomeres possibly escape senescence mechanisms and maintain a proliferative potential after discontinuation of imatinib treatment. To avoid such tumor escape mechanisms, anti-telomerase treatment strategies after TKI discontinuation would seem to be a reasonable proposal.
Session topic: E-poster
Keyword(s): Chronic myeloid leukemia, Imatinib, Telomere, Tyrosine kinase inhibitor
Type: Eposter Presentation
Background
Telomere biology has been more extensively studied in chronic myeloid leukemia (CML) than in any other blood cancer. Shorter telomeres have been associated to disease progression, poor prognosis, higher Hasford score and acquisition of further cytogenetic aberrations. So far, there are no studies investigating the possible influence of telomeres on treatment-free remission (TFR) after discontinuation of therapy with tyrosine kinase inhibitors (TKIs).
Aims
The aim of the present study was to investigate whether telomere length was associated with durable TFR in a cohort of CML patients after discontinuation of treatment with imatinib.
Methods
Thirty-two chronic-phase CML patients discontinued TKI treatment after achieving complete molecular remission (MR4). All patients were treated with imatinib. Two patients underwent second-line TKI (nilotinib) following molecular relapse. Telomere length analysis was performed as described by Cawthon (2002) using a quantitative PCR (q-PCR). The Relative Telomere Length (RTL) was determined as the Telomere (T) to Single copy gene (36B4) (S) ratio (T/S) normalized to a reference sample (K-562 DNA). Peripheral blood samples were also collected from 32 age- and sex-matched healthy controls. Age corrected RTL (acRTL) represented the difference in telomere length between patients and age- and sex-matched controls.
Results
Complete molecular remission (CMR) was achieved after a mean of 26 months of imatinib (range 3-93). TKI treatment was discontinued after a mean of 84 months from the start of treatment (range 24-143). The median follow-up after discontinuation was 30 months (range 18-60). Thirteen patients (41%) showed loss of CMR. All relapsed patients regained CMR after restarting treatment with TKIs. The 36-month cumulative probability of TFR was 59.4%. RTL was assessed at a mean of 32 months from discontinuation in TFR patients and at a mean of 23 months after relapse in others. The median value of acRTL in the CML cohort was 0.09 (range -0.26, +0.86). The Mann-Whitney U test showed shorter acRTL in TFR patients compared to patients with molecular relapse (mean±SD = 0.01±0.14 vs 0.20±0.21; p=0.01). Patients were stratified according to the median value of acRTL ≤0.09. TFR was significantly higher in CML patients with acRTL ≤0.09 in comparison to those with longer telomeres (78.9% vs 30.8%, p=0.002) (Figure).
Conclusion
Achievement of biological recovery through control or eradication of CML stem cells might depend on their proliferative potential in relation to telomere length. Age (a parameter directly proportional to telomere shortening and senescence) has recently been reported as a predictive factor for molecular relapse in CML patients who discontinue imatinib. CML cells would escape senescence through up-regulation of telomerase, restoring telomere length. In our study cohort, TFR patients showed significantly shorter acRTL than patients who presented molecular relapse. It can be postulated that quiescent CML stem cells harboring longer telomeres possibly escape senescence mechanisms and maintain a proliferative potential after discontinuation of imatinib treatment. To avoid such tumor escape mechanisms, anti-telomerase treatment strategies after TKI discontinuation would seem to be a reasonable proposal.

Session topic: E-poster
Keyword(s): Chronic myeloid leukemia, Imatinib, Telomere, Tyrosine kinase inhibitor
Abstract: LB2255
Type: Eposter Presentation
Background
Telomere biology has been more extensively studied in chronic myeloid leukemia (CML) than in any other blood cancer. Shorter telomeres have been associated to disease progression, poor prognosis, higher Hasford score and acquisition of further cytogenetic aberrations. So far, there are no studies investigating the possible influence of telomeres on treatment-free remission (TFR) after discontinuation of therapy with tyrosine kinase inhibitors (TKIs).
Aims
The aim of the present study was to investigate whether telomere length was associated with durable TFR in a cohort of CML patients after discontinuation of treatment with imatinib.
Methods
Thirty-two chronic-phase CML patients discontinued TKI treatment after achieving complete molecular remission (MR4). All patients were treated with imatinib. Two patients underwent second-line TKI (nilotinib) following molecular relapse. Telomere length analysis was performed as described by Cawthon (2002) using a quantitative PCR (q-PCR). The Relative Telomere Length (RTL) was determined as the Telomere (T) to Single copy gene (36B4) (S) ratio (T/S) normalized to a reference sample (K-562 DNA). Peripheral blood samples were also collected from 32 age- and sex-matched healthy controls. Age corrected RTL (acRTL) represented the difference in telomere length between patients and age- and sex-matched controls.
Results
Complete molecular remission (CMR) was achieved after a mean of 26 months of imatinib (range 3-93). TKI treatment was discontinued after a mean of 84 months from the start of treatment (range 24-143). The median follow-up after discontinuation was 30 months (range 18-60). Thirteen patients (41%) showed loss of CMR. All relapsed patients regained CMR after restarting treatment with TKIs. The 36-month cumulative probability of TFR was 59.4%. RTL was assessed at a mean of 32 months from discontinuation in TFR patients and at a mean of 23 months after relapse in others. The median value of acRTL in the CML cohort was 0.09 (range -0.26, +0.86). The Mann-Whitney U test showed shorter acRTL in TFR patients compared to patients with molecular relapse (mean±SD = 0.01±0.14 vs 0.20±0.21; p=0.01). Patients were stratified according to the median value of acRTL ≤0.09. TFR was significantly higher in CML patients with acRTL ≤0.09 in comparison to those with longer telomeres (78.9% vs 30.8%, p=0.002) (Figure).
Conclusion
Achievement of biological recovery through control or eradication of CML stem cells might depend on their proliferative potential in relation to telomere length. Age (a parameter directly proportional to telomere shortening and senescence) has recently been reported as a predictive factor for molecular relapse in CML patients who discontinue imatinib. CML cells would escape senescence through up-regulation of telomerase, restoring telomere length. In our study cohort, TFR patients showed significantly shorter acRTL than patients who presented molecular relapse. It can be postulated that quiescent CML stem cells harboring longer telomeres possibly escape senescence mechanisms and maintain a proliferative potential after discontinuation of imatinib treatment. To avoid such tumor escape mechanisms, anti-telomerase treatment strategies after TKI discontinuation would seem to be a reasonable proposal.

Session topic: E-poster
Keyword(s): Chronic myeloid leukemia, Imatinib, Telomere, Tyrosine kinase inhibitor
Type: Eposter Presentation
Background
Telomere biology has been more extensively studied in chronic myeloid leukemia (CML) than in any other blood cancer. Shorter telomeres have been associated to disease progression, poor prognosis, higher Hasford score and acquisition of further cytogenetic aberrations. So far, there are no studies investigating the possible influence of telomeres on treatment-free remission (TFR) after discontinuation of therapy with tyrosine kinase inhibitors (TKIs).
Aims
The aim of the present study was to investigate whether telomere length was associated with durable TFR in a cohort of CML patients after discontinuation of treatment with imatinib.
Methods
Thirty-two chronic-phase CML patients discontinued TKI treatment after achieving complete molecular remission (MR4). All patients were treated with imatinib. Two patients underwent second-line TKI (nilotinib) following molecular relapse. Telomere length analysis was performed as described by Cawthon (2002) using a quantitative PCR (q-PCR). The Relative Telomere Length (RTL) was determined as the Telomere (T) to Single copy gene (36B4) (S) ratio (T/S) normalized to a reference sample (K-562 DNA). Peripheral blood samples were also collected from 32 age- and sex-matched healthy controls. Age corrected RTL (acRTL) represented the difference in telomere length between patients and age- and sex-matched controls.
Results
Complete molecular remission (CMR) was achieved after a mean of 26 months of imatinib (range 3-93). TKI treatment was discontinued after a mean of 84 months from the start of treatment (range 24-143). The median follow-up after discontinuation was 30 months (range 18-60). Thirteen patients (41%) showed loss of CMR. All relapsed patients regained CMR after restarting treatment with TKIs. The 36-month cumulative probability of TFR was 59.4%. RTL was assessed at a mean of 32 months from discontinuation in TFR patients and at a mean of 23 months after relapse in others. The median value of acRTL in the CML cohort was 0.09 (range -0.26, +0.86). The Mann-Whitney U test showed shorter acRTL in TFR patients compared to patients with molecular relapse (mean±SD = 0.01±0.14 vs 0.20±0.21; p=0.01). Patients were stratified according to the median value of acRTL ≤0.09. TFR was significantly higher in CML patients with acRTL ≤0.09 in comparison to those with longer telomeres (78.9% vs 30.8%, p=0.002) (Figure).
Conclusion
Achievement of biological recovery through control or eradication of CML stem cells might depend on their proliferative potential in relation to telomere length. Age (a parameter directly proportional to telomere shortening and senescence) has recently been reported as a predictive factor for molecular relapse in CML patients who discontinue imatinib. CML cells would escape senescence through up-regulation of telomerase, restoring telomere length. In our study cohort, TFR patients showed significantly shorter acRTL than patients who presented molecular relapse. It can be postulated that quiescent CML stem cells harboring longer telomeres possibly escape senescence mechanisms and maintain a proliferative potential after discontinuation of imatinib treatment. To avoid such tumor escape mechanisms, anti-telomerase treatment strategies after TKI discontinuation would seem to be a reasonable proposal.

Session topic: E-poster
Keyword(s): Chronic myeloid leukemia, Imatinib, Telomere, Tyrosine kinase inhibitor
{{ help_message }}
{{filter}}


