TRANSPOSON-MEDIATED GENERATION OF BCR-ABL1-EXPRESSING TRANSGENIC CELL LINES FOR FAST AND UNBIASED SENSITIVITY TESTING OF TYROSINE KINASE INHIBITORS
(Abstract release date: 05/19/16)
EHA Library. Byrgazov K. 06/09/16; 135364; LB2253

Dr. Konstantin Byrgazov
Contributions
Contributions
Abstract
Abstract: LB2253
Type: Eposter Presentation
Background
Point mutations in the ABL1 kinase domain are an important mechanism of resistance to tyrosine kinase inhibitors (TKI) in BCR-ABL1-positive and, as recently shown, BCR-ABL1-like leukemias. The cell line Ba/F3 lentivirally transduced with mutant BCR-ABL1 constructs is widely used for in vitro sensitivity testing and response prediction to tyrosine kinase inhibitors. The transposon-based Sleeping Beauty system presented offers several advantages over lentiviral transduction including the absence of biosafety issues, faster generation of transgenic cell lines, and greater efficacy in introducing large gene constructs. Nevertheless, both methods can mediate multiple insertions in the genome.
Aims
Establish a method for enrichment of virtually pure cell fractions carrying single insertions of the gene construct to permit unbiased in vitro testing of drug resistance.
Methods
Transposon-mediated gene transfer, flow soritng, FISH, RQ-PCR, qPCR, immunoblotting, survival assays
Results
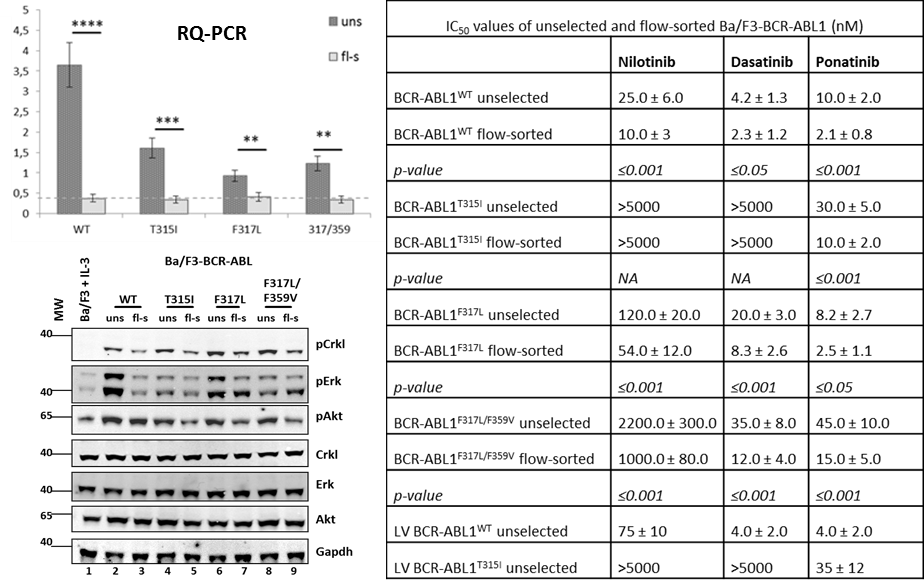
We showed that multiple BCR-ABL1 insertions result in elevated IC50 levels for individual TKIs, thus overestimating the actual resistance of mutant subclones. The establishment of a flow-sorting-based fractionation of BCR-ABL1-transformed Ba/F3 cells has enabled us to enrich for cells carrying single-site insertions, as demonstrated by FISH analysis and real time PCR. Fractions of unselected Ba/F3 cells not only showed a greater number of BCR-ABL1 hybridization signals, higher expression levels of BCR-ABL1, and increased activation of BCR-ABL1 downstream signaling, but also revealed higher IC50 values for the TKIs tested.
Conclusion
The generation of multiple gene construct insertions in Ba/F3 cells by lentiviral- or transposon-mediated transfer has not been reported previously as a relevant problem in the context of in vitro drug sensitivity testing. Our data demonstrate that targeted flow-sorting of low-fluorescent Ba/F3-BCR-ABL1 cells facilitates enrichment of virtually pure cell fractions carrying single insertions of the gene construct. Employment of this selection step is highly recommended to permit unbiased in vitro testing of drug resistance. The clinical relevance of our observations has far reaching consequences beyond Ph+ neoplasia. The rapidly increasing number of reports on BCR-ABL1-like leukemias involving a variety of activated kinases highlights the relevance of TKI-based treatment and in vitro prediction of sensitivity to these agents. Appropriate modeling of mutant kinases inserted at single sites into Ba/F3 cells, as presented in this report, is therefore of paramount importance for reliable prediction of treatment responses to kinase inhibitors.
Session topic: E-poster
Keyword(s): BCR-ABL, Cell line, Drug sensitivity
Type: Eposter Presentation
Background
Point mutations in the ABL1 kinase domain are an important mechanism of resistance to tyrosine kinase inhibitors (TKI) in BCR-ABL1-positive and, as recently shown, BCR-ABL1-like leukemias. The cell line Ba/F3 lentivirally transduced with mutant BCR-ABL1 constructs is widely used for in vitro sensitivity testing and response prediction to tyrosine kinase inhibitors. The transposon-based Sleeping Beauty system presented offers several advantages over lentiviral transduction including the absence of biosafety issues, faster generation of transgenic cell lines, and greater efficacy in introducing large gene constructs. Nevertheless, both methods can mediate multiple insertions in the genome.
Aims
Establish a method for enrichment of virtually pure cell fractions carrying single insertions of the gene construct to permit unbiased in vitro testing of drug resistance.
Methods
Transposon-mediated gene transfer, flow soritng, FISH, RQ-PCR, qPCR, immunoblotting, survival assays
Results
We showed that multiple BCR-ABL1 insertions result in elevated IC50 levels for individual TKIs, thus overestimating the actual resistance of mutant subclones. The establishment of a flow-sorting-based fractionation of BCR-ABL1-transformed Ba/F3 cells has enabled us to enrich for cells carrying single-site insertions, as demonstrated by FISH analysis and real time PCR. Fractions of unselected Ba/F3 cells not only showed a greater number of BCR-ABL1 hybridization signals, higher expression levels of BCR-ABL1, and increased activation of BCR-ABL1 downstream signaling, but also revealed higher IC50 values for the TKIs tested.
Conclusion
The generation of multiple gene construct insertions in Ba/F3 cells by lentiviral- or transposon-mediated transfer has not been reported previously as a relevant problem in the context of in vitro drug sensitivity testing. Our data demonstrate that targeted flow-sorting of low-fluorescent Ba/F3-BCR-ABL1 cells facilitates enrichment of virtually pure cell fractions carrying single insertions of the gene construct. Employment of this selection step is highly recommended to permit unbiased in vitro testing of drug resistance. The clinical relevance of our observations has far reaching consequences beyond Ph+ neoplasia. The rapidly increasing number of reports on BCR-ABL1-like leukemias involving a variety of activated kinases highlights the relevance of TKI-based treatment and in vitro prediction of sensitivity to these agents. Appropriate modeling of mutant kinases inserted at single sites into Ba/F3 cells, as presented in this report, is therefore of paramount importance for reliable prediction of treatment responses to kinase inhibitors.

Session topic: E-poster
Keyword(s): BCR-ABL, Cell line, Drug sensitivity
Abstract: LB2253
Type: Eposter Presentation
Background
Point mutations in the ABL1 kinase domain are an important mechanism of resistance to tyrosine kinase inhibitors (TKI) in BCR-ABL1-positive and, as recently shown, BCR-ABL1-like leukemias. The cell line Ba/F3 lentivirally transduced with mutant BCR-ABL1 constructs is widely used for in vitro sensitivity testing and response prediction to tyrosine kinase inhibitors. The transposon-based Sleeping Beauty system presented offers several advantages over lentiviral transduction including the absence of biosafety issues, faster generation of transgenic cell lines, and greater efficacy in introducing large gene constructs. Nevertheless, both methods can mediate multiple insertions in the genome.
Aims
Establish a method for enrichment of virtually pure cell fractions carrying single insertions of the gene construct to permit unbiased in vitro testing of drug resistance.
Methods
Transposon-mediated gene transfer, flow soritng, FISH, RQ-PCR, qPCR, immunoblotting, survival assays
Results
We showed that multiple BCR-ABL1 insertions result in elevated IC50 levels for individual TKIs, thus overestimating the actual resistance of mutant subclones. The establishment of a flow-sorting-based fractionation of BCR-ABL1-transformed Ba/F3 cells has enabled us to enrich for cells carrying single-site insertions, as demonstrated by FISH analysis and real time PCR. Fractions of unselected Ba/F3 cells not only showed a greater number of BCR-ABL1 hybridization signals, higher expression levels of BCR-ABL1, and increased activation of BCR-ABL1 downstream signaling, but also revealed higher IC50 values for the TKIs tested.
Conclusion
The generation of multiple gene construct insertions in Ba/F3 cells by lentiviral- or transposon-mediated transfer has not been reported previously as a relevant problem in the context of in vitro drug sensitivity testing. Our data demonstrate that targeted flow-sorting of low-fluorescent Ba/F3-BCR-ABL1 cells facilitates enrichment of virtually pure cell fractions carrying single insertions of the gene construct. Employment of this selection step is highly recommended to permit unbiased in vitro testing of drug resistance. The clinical relevance of our observations has far reaching consequences beyond Ph+ neoplasia. The rapidly increasing number of reports on BCR-ABL1-like leukemias involving a variety of activated kinases highlights the relevance of TKI-based treatment and in vitro prediction of sensitivity to these agents. Appropriate modeling of mutant kinases inserted at single sites into Ba/F3 cells, as presented in this report, is therefore of paramount importance for reliable prediction of treatment responses to kinase inhibitors.

Session topic: E-poster
Keyword(s): BCR-ABL, Cell line, Drug sensitivity
Type: Eposter Presentation
Background
Point mutations in the ABL1 kinase domain are an important mechanism of resistance to tyrosine kinase inhibitors (TKI) in BCR-ABL1-positive and, as recently shown, BCR-ABL1-like leukemias. The cell line Ba/F3 lentivirally transduced with mutant BCR-ABL1 constructs is widely used for in vitro sensitivity testing and response prediction to tyrosine kinase inhibitors. The transposon-based Sleeping Beauty system presented offers several advantages over lentiviral transduction including the absence of biosafety issues, faster generation of transgenic cell lines, and greater efficacy in introducing large gene constructs. Nevertheless, both methods can mediate multiple insertions in the genome.
Aims
Establish a method for enrichment of virtually pure cell fractions carrying single insertions of the gene construct to permit unbiased in vitro testing of drug resistance.
Methods
Transposon-mediated gene transfer, flow soritng, FISH, RQ-PCR, qPCR, immunoblotting, survival assays
Results
We showed that multiple BCR-ABL1 insertions result in elevated IC50 levels for individual TKIs, thus overestimating the actual resistance of mutant subclones. The establishment of a flow-sorting-based fractionation of BCR-ABL1-transformed Ba/F3 cells has enabled us to enrich for cells carrying single-site insertions, as demonstrated by FISH analysis and real time PCR. Fractions of unselected Ba/F3 cells not only showed a greater number of BCR-ABL1 hybridization signals, higher expression levels of BCR-ABL1, and increased activation of BCR-ABL1 downstream signaling, but also revealed higher IC50 values for the TKIs tested.
Conclusion
The generation of multiple gene construct insertions in Ba/F3 cells by lentiviral- or transposon-mediated transfer has not been reported previously as a relevant problem in the context of in vitro drug sensitivity testing. Our data demonstrate that targeted flow-sorting of low-fluorescent Ba/F3-BCR-ABL1 cells facilitates enrichment of virtually pure cell fractions carrying single insertions of the gene construct. Employment of this selection step is highly recommended to permit unbiased in vitro testing of drug resistance. The clinical relevance of our observations has far reaching consequences beyond Ph+ neoplasia. The rapidly increasing number of reports on BCR-ABL1-like leukemias involving a variety of activated kinases highlights the relevance of TKI-based treatment and in vitro prediction of sensitivity to these agents. Appropriate modeling of mutant kinases inserted at single sites into Ba/F3 cells, as presented in this report, is therefore of paramount importance for reliable prediction of treatment responses to kinase inhibitors.

Session topic: E-poster
Keyword(s): BCR-ABL, Cell line, Drug sensitivity
{{ help_message }}
{{filter}}


