A PHASE 1 MULTIPLE-DOSE CLINICAL STUDY OF RA101495, A SUBCUTANEOUSLY ADMINISTERED SYNTHETIC MACROCYCLIC PEPTIDE INHIBITOR OF COMPLEMENT C5 FOR TREATMENT OF PAROXYSMAL NOCTURNAL HEMOGLOBINURIA
(Abstract release date: 05/19/16)
EHA Library. Johnston J. 06/09/16; 135360; LB2249
Disclosure(s): Employment by Ra Pharmaceuticals

Dr. Jeffrey Johnston
Contributions
Contributions
Abstract
Abstract: LB2249
Type: Eposter Presentation
Background
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, acquired, clonal hematopoietic stem cell disorder caused by a deficiency in glycosylphosphatidylinositol (GPI)-linked proteins on cell surfaces. Patients with mutations in the phosphatidylinositol glycan class A gene are unable to produce functional, protective, GPI-linked proteins, resulting in the accumulation of specific complement proteins on the surface of red blood cells (RBCs) and subsequent RBC lysis by the membrane attack complex (MAC). Inhibition of complement activation at the level of complement C5 is a clinically validated approach for the treatment of PNH. RA101495, a synthetic macrocyclic peptide, binds to C5 at a unique site not targeted by currently available therapies and allosterically inhibits C5 cleavage into C5a and C5b, preventing production of a key component of the MAC. RA101495 also inhibits the assembly of MAC by blocking the interaction between C5b and C6.
Aims
A Phase 1 multiple-dose clinical pharmacology study in healthy human volunteers designed to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of RA101495 following once daily subcutaneous (SC) injections over 7 days.
Methods
The study was single-center (Australia), randomized, double-blinded, and placebo (PBO)-controlled. After obtaining written informed consent, all subjects received daily SC doses of 0.2 mg/kg RA101495 or matching PBO for 7 days while housed in a clinical pharmacology unit. Safety was assessed by intensive clinical monitoring, and daily blood samples were obtained at -15 minutes, 3 hours, and 6 hours relative to each day’s dose for determination of RA101495 concentrations by liquid chromatography/high resolution mass spectroscopy and ability to inhibit complement-mediated RBC lysis in an ex vivo antibody-sensitized sheep erythrocyte hemolysis assay. All subjects received prophylaxis for N. meningitides infection with ciprofloxacin and vaccination.
Results
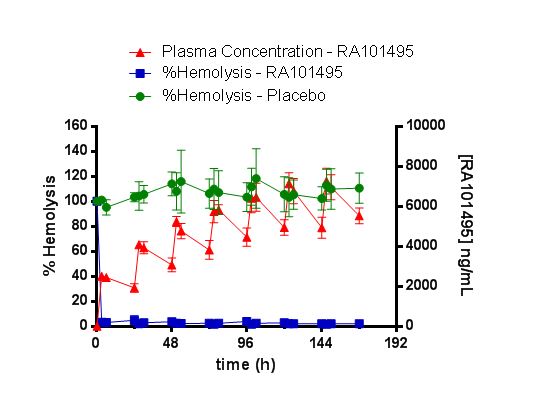
A total of 6 subjects were enrolled into the study (4 RA101495 and 2 PBO). Plasma concentrations showed a steadily increasing exposure over the 7 days of dosing in the 4 RA101495-treated subjects (see Figure). From these data, the half-life of RA101495 appears to be 7 days. Preliminary results show that, for the subjects with measurable plasma levels of RA101495, the mean percent inhibition of hemolysis compared to baseline reached ≥95% beginning at the first time point, 3 hours after dosing on Day 1, and continued throughout the 7 days of dosing; all individual subjects showed ≥90% reduction of hemolysis at all time points (Figure). The only safety finding noted was mild cutaneous injection site erythema in 2 of the 4 RA101495-treated subjects; there was no associated pain, tenderness, swelling, or induration and all events resolved rapidly following dosing.
Conclusion
RA101495 is a novel synthetic macrocyclic peptide inhibitor of C5-mediated hemolysis that is being developed as an alternative to intravenous monoclonal antibody therapy for the treatment of PNH. RA101495, currently being investigated for daily at-home SC self-administration, shows a rapid onset of activity, and appears to be safe and well tolerated. These preliminary data suggest low daily doses will achieve steady-state levels suitable for complete and sustained inhibition of complement and suppression of hemolysis, and, given the long half-life, that once-weekly dosing is possible.
Session topic: E-poster
Keyword(s): Clinical trial, Complement, Paroxysmal nocturnal hemoglobinuria (PNH), Peptide
Type: Eposter Presentation
Background
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, acquired, clonal hematopoietic stem cell disorder caused by a deficiency in glycosylphosphatidylinositol (GPI)-linked proteins on cell surfaces. Patients with mutations in the phosphatidylinositol glycan class A gene are unable to produce functional, protective, GPI-linked proteins, resulting in the accumulation of specific complement proteins on the surface of red blood cells (RBCs) and subsequent RBC lysis by the membrane attack complex (MAC). Inhibition of complement activation at the level of complement C5 is a clinically validated approach for the treatment of PNH. RA101495, a synthetic macrocyclic peptide, binds to C5 at a unique site not targeted by currently available therapies and allosterically inhibits C5 cleavage into C5a and C5b, preventing production of a key component of the MAC. RA101495 also inhibits the assembly of MAC by blocking the interaction between C5b and C6.
Aims
A Phase 1 multiple-dose clinical pharmacology study in healthy human volunteers designed to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of RA101495 following once daily subcutaneous (SC) injections over 7 days.
Methods
The study was single-center (Australia), randomized, double-blinded, and placebo (PBO)-controlled. After obtaining written informed consent, all subjects received daily SC doses of 0.2 mg/kg RA101495 or matching PBO for 7 days while housed in a clinical pharmacology unit. Safety was assessed by intensive clinical monitoring, and daily blood samples were obtained at -15 minutes, 3 hours, and 6 hours relative to each day’s dose for determination of RA101495 concentrations by liquid chromatography/high resolution mass spectroscopy and ability to inhibit complement-mediated RBC lysis in an ex vivo antibody-sensitized sheep erythrocyte hemolysis assay. All subjects received prophylaxis for N. meningitides infection with ciprofloxacin and vaccination.
Results
A total of 6 subjects were enrolled into the study (4 RA101495 and 2 PBO). Plasma concentrations showed a steadily increasing exposure over the 7 days of dosing in the 4 RA101495-treated subjects (see Figure). From these data, the half-life of RA101495 appears to be 7 days. Preliminary results show that, for the subjects with measurable plasma levels of RA101495, the mean percent inhibition of hemolysis compared to baseline reached ≥95% beginning at the first time point, 3 hours after dosing on Day 1, and continued throughout the 7 days of dosing; all individual subjects showed ≥90% reduction of hemolysis at all time points (Figure). The only safety finding noted was mild cutaneous injection site erythema in 2 of the 4 RA101495-treated subjects; there was no associated pain, tenderness, swelling, or induration and all events resolved rapidly following dosing.
Conclusion
RA101495 is a novel synthetic macrocyclic peptide inhibitor of C5-mediated hemolysis that is being developed as an alternative to intravenous monoclonal antibody therapy for the treatment of PNH. RA101495, currently being investigated for daily at-home SC self-administration, shows a rapid onset of activity, and appears to be safe and well tolerated. These preliminary data suggest low daily doses will achieve steady-state levels suitable for complete and sustained inhibition of complement and suppression of hemolysis, and, given the long half-life, that once-weekly dosing is possible.

Session topic: E-poster
Keyword(s): Clinical trial, Complement, Paroxysmal nocturnal hemoglobinuria (PNH), Peptide
Abstract: LB2249
Type: Eposter Presentation
Background
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, acquired, clonal hematopoietic stem cell disorder caused by a deficiency in glycosylphosphatidylinositol (GPI)-linked proteins on cell surfaces. Patients with mutations in the phosphatidylinositol glycan class A gene are unable to produce functional, protective, GPI-linked proteins, resulting in the accumulation of specific complement proteins on the surface of red blood cells (RBCs) and subsequent RBC lysis by the membrane attack complex (MAC). Inhibition of complement activation at the level of complement C5 is a clinically validated approach for the treatment of PNH. RA101495, a synthetic macrocyclic peptide, binds to C5 at a unique site not targeted by currently available therapies and allosterically inhibits C5 cleavage into C5a and C5b, preventing production of a key component of the MAC. RA101495 also inhibits the assembly of MAC by blocking the interaction between C5b and C6.
Aims
A Phase 1 multiple-dose clinical pharmacology study in healthy human volunteers designed to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of RA101495 following once daily subcutaneous (SC) injections over 7 days.
Methods
The study was single-center (Australia), randomized, double-blinded, and placebo (PBO)-controlled. After obtaining written informed consent, all subjects received daily SC doses of 0.2 mg/kg RA101495 or matching PBO for 7 days while housed in a clinical pharmacology unit. Safety was assessed by intensive clinical monitoring, and daily blood samples were obtained at -15 minutes, 3 hours, and 6 hours relative to each day’s dose for determination of RA101495 concentrations by liquid chromatography/high resolution mass spectroscopy and ability to inhibit complement-mediated RBC lysis in an ex vivo antibody-sensitized sheep erythrocyte hemolysis assay. All subjects received prophylaxis for N. meningitides infection with ciprofloxacin and vaccination.
Results
A total of 6 subjects were enrolled into the study (4 RA101495 and 2 PBO). Plasma concentrations showed a steadily increasing exposure over the 7 days of dosing in the 4 RA101495-treated subjects (see Figure). From these data, the half-life of RA101495 appears to be 7 days. Preliminary results show that, for the subjects with measurable plasma levels of RA101495, the mean percent inhibition of hemolysis compared to baseline reached ≥95% beginning at the first time point, 3 hours after dosing on Day 1, and continued throughout the 7 days of dosing; all individual subjects showed ≥90% reduction of hemolysis at all time points (Figure). The only safety finding noted was mild cutaneous injection site erythema in 2 of the 4 RA101495-treated subjects; there was no associated pain, tenderness, swelling, or induration and all events resolved rapidly following dosing.
Conclusion
RA101495 is a novel synthetic macrocyclic peptide inhibitor of C5-mediated hemolysis that is being developed as an alternative to intravenous monoclonal antibody therapy for the treatment of PNH. RA101495, currently being investigated for daily at-home SC self-administration, shows a rapid onset of activity, and appears to be safe and well tolerated. These preliminary data suggest low daily doses will achieve steady-state levels suitable for complete and sustained inhibition of complement and suppression of hemolysis, and, given the long half-life, that once-weekly dosing is possible.

Session topic: E-poster
Keyword(s): Clinical trial, Complement, Paroxysmal nocturnal hemoglobinuria (PNH), Peptide
Type: Eposter Presentation
Background
Paroxysmal nocturnal hemoglobinuria (PNH) is a rare, acquired, clonal hematopoietic stem cell disorder caused by a deficiency in glycosylphosphatidylinositol (GPI)-linked proteins on cell surfaces. Patients with mutations in the phosphatidylinositol glycan class A gene are unable to produce functional, protective, GPI-linked proteins, resulting in the accumulation of specific complement proteins on the surface of red blood cells (RBCs) and subsequent RBC lysis by the membrane attack complex (MAC). Inhibition of complement activation at the level of complement C5 is a clinically validated approach for the treatment of PNH. RA101495, a synthetic macrocyclic peptide, binds to C5 at a unique site not targeted by currently available therapies and allosterically inhibits C5 cleavage into C5a and C5b, preventing production of a key component of the MAC. RA101495 also inhibits the assembly of MAC by blocking the interaction between C5b and C6.
Aims
A Phase 1 multiple-dose clinical pharmacology study in healthy human volunteers designed to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of RA101495 following once daily subcutaneous (SC) injections over 7 days.
Methods
The study was single-center (Australia), randomized, double-blinded, and placebo (PBO)-controlled. After obtaining written informed consent, all subjects received daily SC doses of 0.2 mg/kg RA101495 or matching PBO for 7 days while housed in a clinical pharmacology unit. Safety was assessed by intensive clinical monitoring, and daily blood samples were obtained at -15 minutes, 3 hours, and 6 hours relative to each day’s dose for determination of RA101495 concentrations by liquid chromatography/high resolution mass spectroscopy and ability to inhibit complement-mediated RBC lysis in an ex vivo antibody-sensitized sheep erythrocyte hemolysis assay. All subjects received prophylaxis for N. meningitides infection with ciprofloxacin and vaccination.
Results
A total of 6 subjects were enrolled into the study (4 RA101495 and 2 PBO). Plasma concentrations showed a steadily increasing exposure over the 7 days of dosing in the 4 RA101495-treated subjects (see Figure). From these data, the half-life of RA101495 appears to be 7 days. Preliminary results show that, for the subjects with measurable plasma levels of RA101495, the mean percent inhibition of hemolysis compared to baseline reached ≥95% beginning at the first time point, 3 hours after dosing on Day 1, and continued throughout the 7 days of dosing; all individual subjects showed ≥90% reduction of hemolysis at all time points (Figure). The only safety finding noted was mild cutaneous injection site erythema in 2 of the 4 RA101495-treated subjects; there was no associated pain, tenderness, swelling, or induration and all events resolved rapidly following dosing.
Conclusion
RA101495 is a novel synthetic macrocyclic peptide inhibitor of C5-mediated hemolysis that is being developed as an alternative to intravenous monoclonal antibody therapy for the treatment of PNH. RA101495, currently being investigated for daily at-home SC self-administration, shows a rapid onset of activity, and appears to be safe and well tolerated. These preliminary data suggest low daily doses will achieve steady-state levels suitable for complete and sustained inhibition of complement and suppression of hemolysis, and, given the long half-life, that once-weekly dosing is possible.

Session topic: E-poster
Keyword(s): Clinical trial, Complement, Paroxysmal nocturnal hemoglobinuria (PNH), Peptide
{{ help_message }}
{{filter}}


