FRONT-LINE CENTRAL VASCULAR ACCESS DEVICES IN ACUTE LEUKEMIAS - PERIPHERALLY INSERTED CENTRAL CATHETER (PICC) VERSUS TRADITIONAL CENTRAL VENOUS CATHETER(CVC): A PHASE IV RANDOMIZED TRIAL (NCT02405728)
(Abstract release date: 05/19/16)
EHA Library. Cerchione C. 06/09/16; 135355; LB2244

Dr. Claudio Cerchione
Contributions
Contributions
Abstract
Abstract: LB2244
Type: Eposter Presentation
Background
The use of PICC as an alternative to other CVC devices, particularly for prolonged infusions of cytotoxic agents, blood products and/or other supportive therapy, is becoming very frequent in cancer patients. PICCs are easier to insert, and associated to a lower rate of complications than traditional CVC. However, in the setting of high-risk patients with acute leukemia there is limited information on the feasibility and safety of PICC as primary vascular access device. Our Hematology Department is conducting a Phase IV randomized trial. We compare PICC versus traditional CVC as front-line venous access device in patients with acute leukemias undergoing remission induction chemotherapy. This trial is registered on ClinicalTrials.gov, number NCT02405728 (ongoing).
Aims
Primary endpoint is the occurrence of catheter-related bloodstream infections and/or catheter-related thrombosis. Secondary endpoints are the occurrence of other complications, such as pneumothorax or catheter occlusion, and patient quality of life. Questionnaire covering functional status, sleep and hygiene disturbance had been given to assess patients’quality of life.
Methods
From April 2015 to February 2016,152 patients with acute leukemia planned for remission induction chemotherapy were randomly assigned (1:1) to PICC (Arm A) or traditional CVC (Arm B).Inclusion Criteria were: age >18 years, suspected survival > 4 weeks, and need of central venous access device (long-term; > 4 weeks). While exclusion criteria were ongoing uncontrolled systemic infection, presence of significant thrombosis/stenosis in arm or central veins, and inability to communicate and/or to sign informed consent. All insertions were followed by chest X-ray.
Results
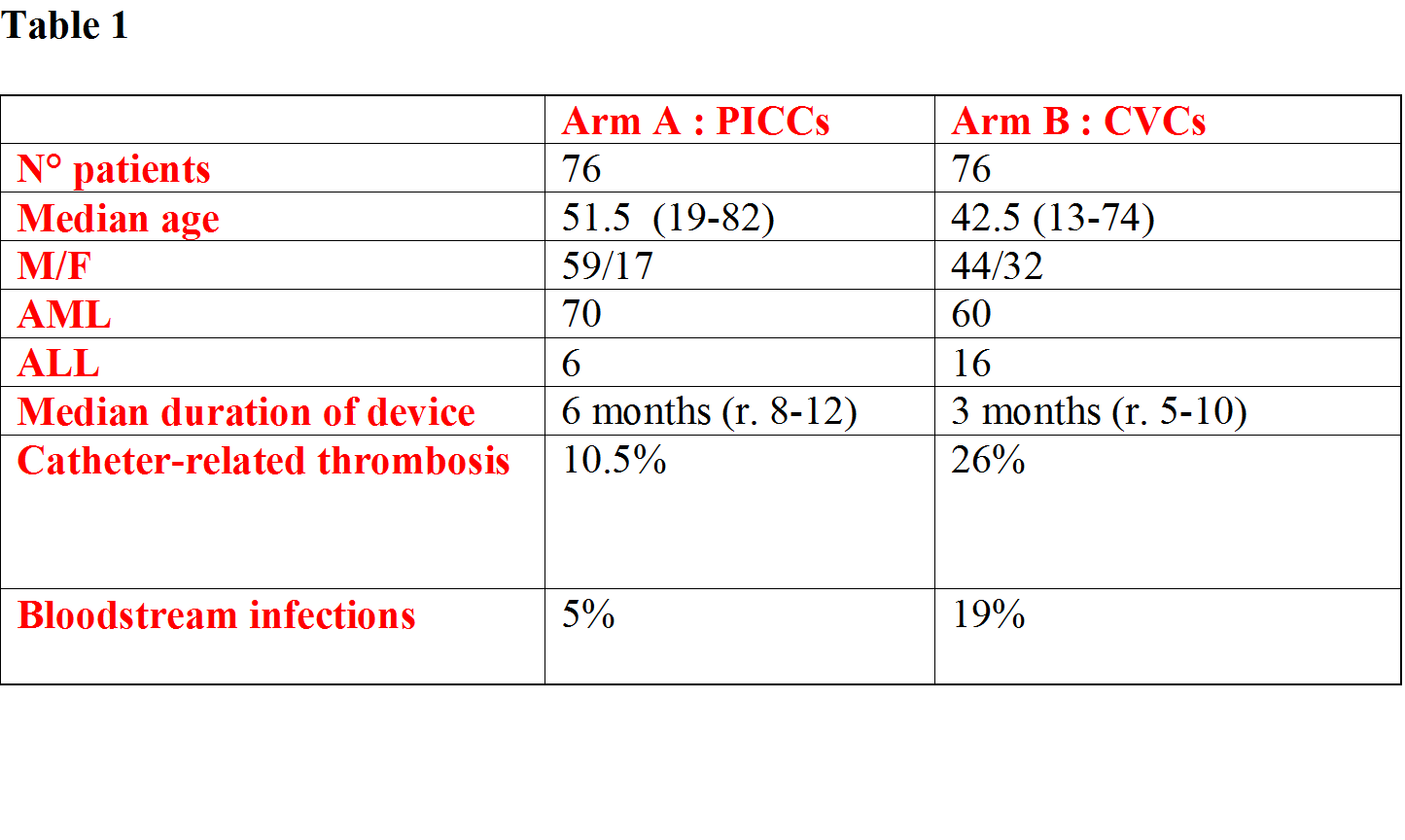
152 patients (130 AML, 22 ALL), median age 47 years (13-82), were randomized in the two arms. In Arm A, 76 PICCs were inserted in 76 hematological patients (median age 51.5 years, r.19-82, 17 females, 59 males) suffering from acute myeloid leukemia (AML : 70) or acute lymphoblastic leukemia (ALL : 6). Double lumen PICCs (5 Fr) were inserted in 70 patients, single lumen PICCs (4 Fr) were inserted in 5 patients, and triple lumen PICC (6Fr) was inserted in 1 patient.68 PICCs were inserted in the right basilica vein, 5 PICCs were inserted in the left basilica vein and 3 PICCs were inserted in the left brachial vein.In Arm B, 76 traditional CVCs were inserted in other 76 hematological patients (44 males, 32 females) suffering from AML (60) or ALL (16). 45 CVCs were inserted in subclavian vein and 31 CVCs were inserted in jugular vein.Overall, the median duration of in situ catheter placement was 5 months. In the arm A, median duration of device was 6 months (r. 12-8), catheter-related thrombosis occurred in 8 patients (6 basilica vein, 2 brachial vein) and catheter-related bloodstream infections in 4 patients (2 methicillin-resistant, 1 coagulase-negative, 1 staphylococci).In the arm B, median duration of device was 3 months (r. 5-10), 20 cases of catheter-related thrombosis (7 subclavian vein, 13 jugular vein) and 15 cases of catheter-related bloodstream infections (3 methicillin-resistant, 2 coagulase-negative, 4 staphylococci, 6 enterobacteriaceae) were observed.Thus, PICCs were significantly associated with fewer major complications compared with traditional CVC (catheter-related thrombosis: 10.5% in the arm A vs. 26% in the arm B, P=0.01 by χ2 test; catheter-related bloodstream infections: 5% in the arm A vs. 19% in the arm B, P= 0.007 by χ2 test).
Conclusion
The preliminary observations of this ongoing Phase IV randomized study, focusing on front-line use of central venous access device, suggest that the use of PICC represents an advance in terms of decrease of complication rate and improve of quality of life for patients with acute leukemia.
Session topic: E-poster
Keyword(s): Acute leukemia, Catheter-related thrombosis, Infection
Type: Eposter Presentation
Background
The use of PICC as an alternative to other CVC devices, particularly for prolonged infusions of cytotoxic agents, blood products and/or other supportive therapy, is becoming very frequent in cancer patients. PICCs are easier to insert, and associated to a lower rate of complications than traditional CVC. However, in the setting of high-risk patients with acute leukemia there is limited information on the feasibility and safety of PICC as primary vascular access device. Our Hematology Department is conducting a Phase IV randomized trial. We compare PICC versus traditional CVC as front-line venous access device in patients with acute leukemias undergoing remission induction chemotherapy. This trial is registered on ClinicalTrials.gov, number NCT02405728 (ongoing).
Aims
Primary endpoint is the occurrence of catheter-related bloodstream infections and/or catheter-related thrombosis. Secondary endpoints are the occurrence of other complications, such as pneumothorax or catheter occlusion, and patient quality of life. Questionnaire covering functional status, sleep and hygiene disturbance had been given to assess patients’quality of life.
Methods
From April 2015 to February 2016,152 patients with acute leukemia planned for remission induction chemotherapy were randomly assigned (1:1) to PICC (Arm A) or traditional CVC (Arm B).Inclusion Criteria were: age >18 years, suspected survival > 4 weeks, and need of central venous access device (long-term; > 4 weeks). While exclusion criteria were ongoing uncontrolled systemic infection, presence of significant thrombosis/stenosis in arm or central veins, and inability to communicate and/or to sign informed consent. All insertions were followed by chest X-ray.
Results
152 patients (130 AML, 22 ALL), median age 47 years (13-82), were randomized in the two arms. In Arm A, 76 PICCs were inserted in 76 hematological patients (median age 51.5 years, r.19-82, 17 females, 59 males) suffering from acute myeloid leukemia (AML : 70) or acute lymphoblastic leukemia (ALL : 6). Double lumen PICCs (5 Fr) were inserted in 70 patients, single lumen PICCs (4 Fr) were inserted in 5 patients, and triple lumen PICC (6Fr) was inserted in 1 patient.68 PICCs were inserted in the right basilica vein, 5 PICCs were inserted in the left basilica vein and 3 PICCs were inserted in the left brachial vein.In Arm B, 76 traditional CVCs were inserted in other 76 hematological patients (44 males, 32 females) suffering from AML (60) or ALL (16). 45 CVCs were inserted in subclavian vein and 31 CVCs were inserted in jugular vein.Overall, the median duration of in situ catheter placement was 5 months. In the arm A, median duration of device was 6 months (r. 12-8), catheter-related thrombosis occurred in 8 patients (6 basilica vein, 2 brachial vein) and catheter-related bloodstream infections in 4 patients (2 methicillin-resistant, 1 coagulase-negative, 1 staphylococci).In the arm B, median duration of device was 3 months (r. 5-10), 20 cases of catheter-related thrombosis (7 subclavian vein, 13 jugular vein) and 15 cases of catheter-related bloodstream infections (3 methicillin-resistant, 2 coagulase-negative, 4 staphylococci, 6 enterobacteriaceae) were observed.Thus, PICCs were significantly associated with fewer major complications compared with traditional CVC (catheter-related thrombosis: 10.5% in the arm A vs. 26% in the arm B, P=0.01 by χ2 test; catheter-related bloodstream infections: 5% in the arm A vs. 19% in the arm B, P= 0.007 by χ2 test).
Conclusion
The preliminary observations of this ongoing Phase IV randomized study, focusing on front-line use of central venous access device, suggest that the use of PICC represents an advance in terms of decrease of complication rate and improve of quality of life for patients with acute leukemia.

Session topic: E-poster
Keyword(s): Acute leukemia, Catheter-related thrombosis, Infection
Abstract: LB2244
Type: Eposter Presentation
Background
The use of PICC as an alternative to other CVC devices, particularly for prolonged infusions of cytotoxic agents, blood products and/or other supportive therapy, is becoming very frequent in cancer patients. PICCs are easier to insert, and associated to a lower rate of complications than traditional CVC. However, in the setting of high-risk patients with acute leukemia there is limited information on the feasibility and safety of PICC as primary vascular access device. Our Hematology Department is conducting a Phase IV randomized trial. We compare PICC versus traditional CVC as front-line venous access device in patients with acute leukemias undergoing remission induction chemotherapy. This trial is registered on ClinicalTrials.gov, number NCT02405728 (ongoing).
Aims
Primary endpoint is the occurrence of catheter-related bloodstream infections and/or catheter-related thrombosis. Secondary endpoints are the occurrence of other complications, such as pneumothorax or catheter occlusion, and patient quality of life. Questionnaire covering functional status, sleep and hygiene disturbance had been given to assess patients’quality of life.
Methods
From April 2015 to February 2016,152 patients with acute leukemia planned for remission induction chemotherapy were randomly assigned (1:1) to PICC (Arm A) or traditional CVC (Arm B).Inclusion Criteria were: age >18 years, suspected survival > 4 weeks, and need of central venous access device (long-term; > 4 weeks). While exclusion criteria were ongoing uncontrolled systemic infection, presence of significant thrombosis/stenosis in arm or central veins, and inability to communicate and/or to sign informed consent. All insertions were followed by chest X-ray.
Results
152 patients (130 AML, 22 ALL), median age 47 years (13-82), were randomized in the two arms. In Arm A, 76 PICCs were inserted in 76 hematological patients (median age 51.5 years, r.19-82, 17 females, 59 males) suffering from acute myeloid leukemia (AML : 70) or acute lymphoblastic leukemia (ALL : 6). Double lumen PICCs (5 Fr) were inserted in 70 patients, single lumen PICCs (4 Fr) were inserted in 5 patients, and triple lumen PICC (6Fr) was inserted in 1 patient.68 PICCs were inserted in the right basilica vein, 5 PICCs were inserted in the left basilica vein and 3 PICCs were inserted in the left brachial vein.In Arm B, 76 traditional CVCs were inserted in other 76 hematological patients (44 males, 32 females) suffering from AML (60) or ALL (16). 45 CVCs were inserted in subclavian vein and 31 CVCs were inserted in jugular vein.Overall, the median duration of in situ catheter placement was 5 months. In the arm A, median duration of device was 6 months (r. 12-8), catheter-related thrombosis occurred in 8 patients (6 basilica vein, 2 brachial vein) and catheter-related bloodstream infections in 4 patients (2 methicillin-resistant, 1 coagulase-negative, 1 staphylococci).In the arm B, median duration of device was 3 months (r. 5-10), 20 cases of catheter-related thrombosis (7 subclavian vein, 13 jugular vein) and 15 cases of catheter-related bloodstream infections (3 methicillin-resistant, 2 coagulase-negative, 4 staphylococci, 6 enterobacteriaceae) were observed.Thus, PICCs were significantly associated with fewer major complications compared with traditional CVC (catheter-related thrombosis: 10.5% in the arm A vs. 26% in the arm B, P=0.01 by χ2 test; catheter-related bloodstream infections: 5% in the arm A vs. 19% in the arm B, P= 0.007 by χ2 test).
Conclusion
The preliminary observations of this ongoing Phase IV randomized study, focusing on front-line use of central venous access device, suggest that the use of PICC represents an advance in terms of decrease of complication rate and improve of quality of life for patients with acute leukemia.

Session topic: E-poster
Keyword(s): Acute leukemia, Catheter-related thrombosis, Infection
Type: Eposter Presentation
Background
The use of PICC as an alternative to other CVC devices, particularly for prolonged infusions of cytotoxic agents, blood products and/or other supportive therapy, is becoming very frequent in cancer patients. PICCs are easier to insert, and associated to a lower rate of complications than traditional CVC. However, in the setting of high-risk patients with acute leukemia there is limited information on the feasibility and safety of PICC as primary vascular access device. Our Hematology Department is conducting a Phase IV randomized trial. We compare PICC versus traditional CVC as front-line venous access device in patients with acute leukemias undergoing remission induction chemotherapy. This trial is registered on ClinicalTrials.gov, number NCT02405728 (ongoing).
Aims
Primary endpoint is the occurrence of catheter-related bloodstream infections and/or catheter-related thrombosis. Secondary endpoints are the occurrence of other complications, such as pneumothorax or catheter occlusion, and patient quality of life. Questionnaire covering functional status, sleep and hygiene disturbance had been given to assess patients’quality of life.
Methods
From April 2015 to February 2016,152 patients with acute leukemia planned for remission induction chemotherapy were randomly assigned (1:1) to PICC (Arm A) or traditional CVC (Arm B).Inclusion Criteria were: age >18 years, suspected survival > 4 weeks, and need of central venous access device (long-term; > 4 weeks). While exclusion criteria were ongoing uncontrolled systemic infection, presence of significant thrombosis/stenosis in arm or central veins, and inability to communicate and/or to sign informed consent. All insertions were followed by chest X-ray.
Results
152 patients (130 AML, 22 ALL), median age 47 years (13-82), were randomized in the two arms. In Arm A, 76 PICCs were inserted in 76 hematological patients (median age 51.5 years, r.19-82, 17 females, 59 males) suffering from acute myeloid leukemia (AML : 70) or acute lymphoblastic leukemia (ALL : 6). Double lumen PICCs (5 Fr) were inserted in 70 patients, single lumen PICCs (4 Fr) were inserted in 5 patients, and triple lumen PICC (6Fr) was inserted in 1 patient.68 PICCs were inserted in the right basilica vein, 5 PICCs were inserted in the left basilica vein and 3 PICCs were inserted in the left brachial vein.In Arm B, 76 traditional CVCs were inserted in other 76 hematological patients (44 males, 32 females) suffering from AML (60) or ALL (16). 45 CVCs were inserted in subclavian vein and 31 CVCs were inserted in jugular vein.Overall, the median duration of in situ catheter placement was 5 months. In the arm A, median duration of device was 6 months (r. 12-8), catheter-related thrombosis occurred in 8 patients (6 basilica vein, 2 brachial vein) and catheter-related bloodstream infections in 4 patients (2 methicillin-resistant, 1 coagulase-negative, 1 staphylococci).In the arm B, median duration of device was 3 months (r. 5-10), 20 cases of catheter-related thrombosis (7 subclavian vein, 13 jugular vein) and 15 cases of catheter-related bloodstream infections (3 methicillin-resistant, 2 coagulase-negative, 4 staphylococci, 6 enterobacteriaceae) were observed.Thus, PICCs were significantly associated with fewer major complications compared with traditional CVC (catheter-related thrombosis: 10.5% in the arm A vs. 26% in the arm B, P=0.01 by χ2 test; catheter-related bloodstream infections: 5% in the arm A vs. 19% in the arm B, P= 0.007 by χ2 test).
Conclusion
The preliminary observations of this ongoing Phase IV randomized study, focusing on front-line use of central venous access device, suggest that the use of PICC represents an advance in terms of decrease of complication rate and improve of quality of life for patients with acute leukemia.

Session topic: E-poster
Keyword(s): Acute leukemia, Catheter-related thrombosis, Infection
{{ help_message }}
{{filter}}


