PODOPLANIN EXPRESSION AND INTRAVASCULAR PLATELET AGGREGATES: THE MISSING LINK BETWEEN CANCER AND THROMBOSIS IN PRIMARY MALIGNANT BRAIN TUMORS
(Abstract release date: 05/19/16)
EHA Library. Riedl J. 06/10/16; 135173; S140

Dr. Julia Riedl
Contributions
Contributions
Abstract
Abstract: S140
Type: Oral Presentation
Presentation during EHA21: On Friday, June 10, 2016 from 12:30 - 12:45
Location: Room H4
Background
Venous thromboembolism (VTE) is a common clinical problem in patients with primary malignant brain tumors, and underlying mechanisms are unclear.
Aims
In a prospective observational study, we explored the association of podoplanin, a sialomucin-like glycoprotein that has the ability to induce blood platelet activation and aggregation, with VTE in primary malignant brain tumors. Furthermore, we investigated the ability of primary human glioblastoma cells, isolated from a patient with glioblastoma and thrombotic complications, to activate human platelets in-vitro.
Methods
Immunohistochemical (IHC) staining against podoplanin and platelet surface protein CD61 was performed in brain tumor specimens of 213 adult patients (mostly high-grade gliomas [89%]) included in the Vienna Cancer and Thrombosis Study (CATS), a prospective observational cohort study of patients with newly diagnosed cancer or progressive disease. Primary endpoint was symptomatic VTE.In-vitro, co-incubation experiments were performed using primary glioblastoma cells isolated from a patient included in CATS who had developed pulmonary embolism (PE) during follow up and whose tumor stained positive for podoplanin in IHC. Activation and aggregation of human platelets upon co-incubation with different concentrations of cancer cells was investigated by light transmission aggregometry and macro- and microscopical visualization.
Results
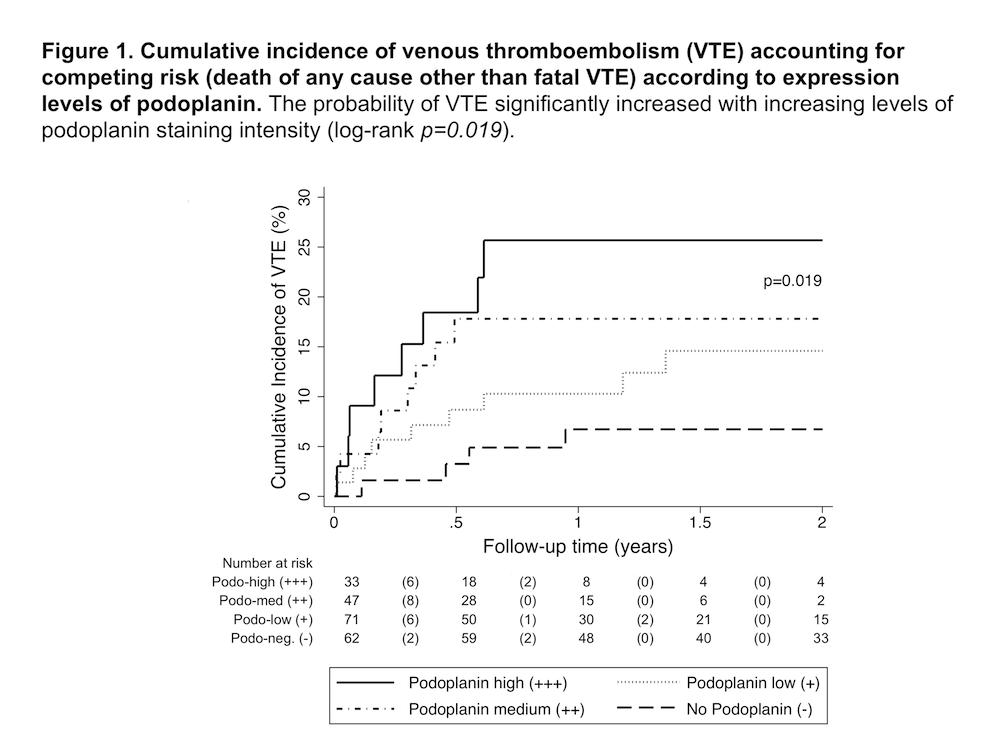
During 2-year-follow-up, 29 (13.6%) patients developed VTE. In total, 151 (70.9%) tumor specimens stained positive for podoplanin (33 high expression, 47 medium expression, 71 low expression). Patients with podoplanin positive tumors had a lower blood platelet count (Median [25th-75th percentile], G/l: 227 [186-285] vs. 286 [241-355]; p<0.001) and higher D-dimer levels (mg/ml: 0.85 [0.46-1.92] vs. 0.42 [0.23-0.79]; p<0.001). Increasing podoplanin-staining intensity was associated with increasing levels of CD61-positive intravascular platelet aggregates in tumor specimens (p<0.001).In Cox regression analysis, high podoplanin expression was associated with increased risk of VTE (hazard ratio [HR] for high vs. no podoplanin expression: 5.75, 95% confidence interval [CI]: 1.71-19.27; p=0.005). This association was independent of age, sex and tumor grade (HR 5.71, 95%CI: 1.52-21.26; p=0.010). Figure 1 shows cumulative incidence curves of VTE according to podoplanin expression levels.Podoplanin-positive glioblastoma cells induced platelet aggregation, measured by light transmission aggregometry, in a dose-dependent manner, which was not observed in a podoplanin-negative control glioblastoma cell line. Podoplanin-positive glioblastoma cells also induced marked macro- and microscopically visible blood coagulation upon co-incubation with whole blood from a healthy donor, while this was not observed for podoplanin-negative control cells.
Conclusion
High podoplanin expression in primary malignant brain tumors, which correlated with intravascular platelet aggregates, lower blood platelet counts and higher D-dimer levels, was associated with increased risk of VTE. In-vitro, podoplanin-positive cancer cells isolated from a glioblastoma patient who developed PE induced marked platelet activation and aggregation. Our study might provide a novel mechanistic insight into the pathogenesis of VTE in patients with primary malignant brain tumors.
Session topic: Thrombosis
Keyword(s): Cancer, Platelet, Platelet aggregation, Venous thromboembolism
Type: Oral Presentation
Presentation during EHA21: On Friday, June 10, 2016 from 12:30 - 12:45
Location: Room H4
Background
Venous thromboembolism (VTE) is a common clinical problem in patients with primary malignant brain tumors, and underlying mechanisms are unclear.
Aims
In a prospective observational study, we explored the association of podoplanin, a sialomucin-like glycoprotein that has the ability to induce blood platelet activation and aggregation, with VTE in primary malignant brain tumors. Furthermore, we investigated the ability of primary human glioblastoma cells, isolated from a patient with glioblastoma and thrombotic complications, to activate human platelets in-vitro.
Methods
Immunohistochemical (IHC) staining against podoplanin and platelet surface protein CD61 was performed in brain tumor specimens of 213 adult patients (mostly high-grade gliomas [89%]) included in the Vienna Cancer and Thrombosis Study (CATS), a prospective observational cohort study of patients with newly diagnosed cancer or progressive disease. Primary endpoint was symptomatic VTE.In-vitro, co-incubation experiments were performed using primary glioblastoma cells isolated from a patient included in CATS who had developed pulmonary embolism (PE) during follow up and whose tumor stained positive for podoplanin in IHC. Activation and aggregation of human platelets upon co-incubation with different concentrations of cancer cells was investigated by light transmission aggregometry and macro- and microscopical visualization.
Results
During 2-year-follow-up, 29 (13.6%) patients developed VTE. In total, 151 (70.9%) tumor specimens stained positive for podoplanin (33 high expression, 47 medium expression, 71 low expression). Patients with podoplanin positive tumors had a lower blood platelet count (Median [25th-75th percentile], G/l: 227 [186-285] vs. 286 [241-355]; p<0.001) and higher D-dimer levels (mg/ml: 0.85 [0.46-1.92] vs. 0.42 [0.23-0.79]; p<0.001). Increasing podoplanin-staining intensity was associated with increasing levels of CD61-positive intravascular platelet aggregates in tumor specimens (p<0.001).In Cox regression analysis, high podoplanin expression was associated with increased risk of VTE (hazard ratio [HR] for high vs. no podoplanin expression: 5.75, 95% confidence interval [CI]: 1.71-19.27; p=0.005). This association was independent of age, sex and tumor grade (HR 5.71, 95%CI: 1.52-21.26; p=0.010). Figure 1 shows cumulative incidence curves of VTE according to podoplanin expression levels.Podoplanin-positive glioblastoma cells induced platelet aggregation, measured by light transmission aggregometry, in a dose-dependent manner, which was not observed in a podoplanin-negative control glioblastoma cell line. Podoplanin-positive glioblastoma cells also induced marked macro- and microscopically visible blood coagulation upon co-incubation with whole blood from a healthy donor, while this was not observed for podoplanin-negative control cells.
Conclusion
High podoplanin expression in primary malignant brain tumors, which correlated with intravascular platelet aggregates, lower blood platelet counts and higher D-dimer levels, was associated with increased risk of VTE. In-vitro, podoplanin-positive cancer cells isolated from a glioblastoma patient who developed PE induced marked platelet activation and aggregation. Our study might provide a novel mechanistic insight into the pathogenesis of VTE in patients with primary malignant brain tumors.

Session topic: Thrombosis
Keyword(s): Cancer, Platelet, Platelet aggregation, Venous thromboembolism
Abstract: S140
Type: Oral Presentation
Presentation during EHA21: On Friday, June 10, 2016 from 12:30 - 12:45
Location: Room H4
Background
Venous thromboembolism (VTE) is a common clinical problem in patients with primary malignant brain tumors, and underlying mechanisms are unclear.
Aims
In a prospective observational study, we explored the association of podoplanin, a sialomucin-like glycoprotein that has the ability to induce blood platelet activation and aggregation, with VTE in primary malignant brain tumors. Furthermore, we investigated the ability of primary human glioblastoma cells, isolated from a patient with glioblastoma and thrombotic complications, to activate human platelets in-vitro.
Methods
Immunohistochemical (IHC) staining against podoplanin and platelet surface protein CD61 was performed in brain tumor specimens of 213 adult patients (mostly high-grade gliomas [89%]) included in the Vienna Cancer and Thrombosis Study (CATS), a prospective observational cohort study of patients with newly diagnosed cancer or progressive disease. Primary endpoint was symptomatic VTE.In-vitro, co-incubation experiments were performed using primary glioblastoma cells isolated from a patient included in CATS who had developed pulmonary embolism (PE) during follow up and whose tumor stained positive for podoplanin in IHC. Activation and aggregation of human platelets upon co-incubation with different concentrations of cancer cells was investigated by light transmission aggregometry and macro- and microscopical visualization.
Results
During 2-year-follow-up, 29 (13.6%) patients developed VTE. In total, 151 (70.9%) tumor specimens stained positive for podoplanin (33 high expression, 47 medium expression, 71 low expression). Patients with podoplanin positive tumors had a lower blood platelet count (Median [25th-75th percentile], G/l: 227 [186-285] vs. 286 [241-355]; p<0.001) and higher D-dimer levels (mg/ml: 0.85 [0.46-1.92] vs. 0.42 [0.23-0.79]; p<0.001). Increasing podoplanin-staining intensity was associated with increasing levels of CD61-positive intravascular platelet aggregates in tumor specimens (p<0.001).In Cox regression analysis, high podoplanin expression was associated with increased risk of VTE (hazard ratio [HR] for high vs. no podoplanin expression: 5.75, 95% confidence interval [CI]: 1.71-19.27; p=0.005). This association was independent of age, sex and tumor grade (HR 5.71, 95%CI: 1.52-21.26; p=0.010). Figure 1 shows cumulative incidence curves of VTE according to podoplanin expression levels.Podoplanin-positive glioblastoma cells induced platelet aggregation, measured by light transmission aggregometry, in a dose-dependent manner, which was not observed in a podoplanin-negative control glioblastoma cell line. Podoplanin-positive glioblastoma cells also induced marked macro- and microscopically visible blood coagulation upon co-incubation with whole blood from a healthy donor, while this was not observed for podoplanin-negative control cells.
Conclusion
High podoplanin expression in primary malignant brain tumors, which correlated with intravascular platelet aggregates, lower blood platelet counts and higher D-dimer levels, was associated with increased risk of VTE. In-vitro, podoplanin-positive cancer cells isolated from a glioblastoma patient who developed PE induced marked platelet activation and aggregation. Our study might provide a novel mechanistic insight into the pathogenesis of VTE in patients with primary malignant brain tumors.

Session topic: Thrombosis
Keyword(s): Cancer, Platelet, Platelet aggregation, Venous thromboembolism
Type: Oral Presentation
Presentation during EHA21: On Friday, June 10, 2016 from 12:30 - 12:45
Location: Room H4
Background
Venous thromboembolism (VTE) is a common clinical problem in patients with primary malignant brain tumors, and underlying mechanisms are unclear.
Aims
In a prospective observational study, we explored the association of podoplanin, a sialomucin-like glycoprotein that has the ability to induce blood platelet activation and aggregation, with VTE in primary malignant brain tumors. Furthermore, we investigated the ability of primary human glioblastoma cells, isolated from a patient with glioblastoma and thrombotic complications, to activate human platelets in-vitro.
Methods
Immunohistochemical (IHC) staining against podoplanin and platelet surface protein CD61 was performed in brain tumor specimens of 213 adult patients (mostly high-grade gliomas [89%]) included in the Vienna Cancer and Thrombosis Study (CATS), a prospective observational cohort study of patients with newly diagnosed cancer or progressive disease. Primary endpoint was symptomatic VTE.In-vitro, co-incubation experiments were performed using primary glioblastoma cells isolated from a patient included in CATS who had developed pulmonary embolism (PE) during follow up and whose tumor stained positive for podoplanin in IHC. Activation and aggregation of human platelets upon co-incubation with different concentrations of cancer cells was investigated by light transmission aggregometry and macro- and microscopical visualization.
Results
During 2-year-follow-up, 29 (13.6%) patients developed VTE. In total, 151 (70.9%) tumor specimens stained positive for podoplanin (33 high expression, 47 medium expression, 71 low expression). Patients with podoplanin positive tumors had a lower blood platelet count (Median [25th-75th percentile], G/l: 227 [186-285] vs. 286 [241-355]; p<0.001) and higher D-dimer levels (mg/ml: 0.85 [0.46-1.92] vs. 0.42 [0.23-0.79]; p<0.001). Increasing podoplanin-staining intensity was associated with increasing levels of CD61-positive intravascular platelet aggregates in tumor specimens (p<0.001).In Cox regression analysis, high podoplanin expression was associated with increased risk of VTE (hazard ratio [HR] for high vs. no podoplanin expression: 5.75, 95% confidence interval [CI]: 1.71-19.27; p=0.005). This association was independent of age, sex and tumor grade (HR 5.71, 95%CI: 1.52-21.26; p=0.010). Figure 1 shows cumulative incidence curves of VTE according to podoplanin expression levels.Podoplanin-positive glioblastoma cells induced platelet aggregation, measured by light transmission aggregometry, in a dose-dependent manner, which was not observed in a podoplanin-negative control glioblastoma cell line. Podoplanin-positive glioblastoma cells also induced marked macro- and microscopically visible blood coagulation upon co-incubation with whole blood from a healthy donor, while this was not observed for podoplanin-negative control cells.
Conclusion
High podoplanin expression in primary malignant brain tumors, which correlated with intravascular platelet aggregates, lower blood platelet counts and higher D-dimer levels, was associated with increased risk of VTE. In-vitro, podoplanin-positive cancer cells isolated from a glioblastoma patient who developed PE induced marked platelet activation and aggregation. Our study might provide a novel mechanistic insight into the pathogenesis of VTE in patients with primary malignant brain tumors.

Session topic: Thrombosis
Keyword(s): Cancer, Platelet, Platelet aggregation, Venous thromboembolism
{{ help_message }}
{{filter}}


