HIGH SYSTEMIC IRON LEVEL IS A RISK FACTOR FOR CARDIOVASCULAR DISEASE
(Abstract release date: 05/19/16)
EHA Library. Vinchi F. 06/10/16; 135167; S134

Dr. Francesca Vinchi
Contributions
Contributions
Abstract
Abstract: S134
Type: Oral Presentation
Presentation during EHA21: On Friday, June 10, 2016 from 11:45 - 12:00
Location: Hall C15
Background
Iron accumulates in atherosclerotic lesions but its role in atherogenesis is still debated. In the “iron hypothesis” (1981), Sullivan proposed that iron is detrimental for the cardiovascular system, promoting atherosclerosis progression. So far, epidemiological data and studies in animal models have provided conflicting evidence regarding a role of excess iron in atherogenesis and cardiovascular disease.
Aims
In this study we aimed to investigate the role of iron overload in the development of atherosclerosis.
Methods
To this purpose, a mouse model of type IV Hereditary Hemochromatosis, in which the hepcidin/ferroportin regulatory circuitry is disrupted due to a point mutation in the iron exporter ferroportin (FPNC326S; Altamura et al., Cell Metabolism 2014), was interbred with ApoE-null mice(ApoE-/-), that show increased susceptibility to atherosclerosis. Plaque formation was analyzed in ApoE-/-FPNwt/C326S mice at 6 and 12 months of age.
Results
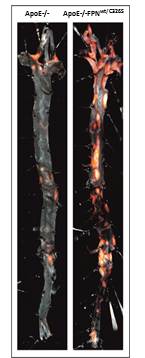
ApoE-/-FPNwt/C326S mice show high serum iron and cholesterol levels, as expected. Importantly, these mice show strongly increased lesion size and number at both 6 and 12 months of age compared to age-matched Apo-/- mice (6 months: 1.44±0.23 vs 5±0.53 % aortic lesion area, P=0.0001; 12 months: 10.24±1.21 vs 20.44±2.69 % aortic lesion area, P=0.0065). The atherosclerotic phenotype positively correlates with higher levels of circulating iron (12 months: 122.2±6.57 vs 337±19.22 mg iron/dl serum, P<0.0001) and oxidized LDLs (12 months: 2151±136.8 vs 3243±193.9 nmol oxLDL/ml serum, P=0.0002).Iron is deposited in the artery media layer, which correlates with vascular smooth muscle cell senescence, calcifications, vascular oxidative stress (12 months: 2.45±0.2 vs 4.33±0.32 nmol MDA/mg aorta protein, P=0.047) and DNA damage. We observe increased vascular permeability (6 months: 0.95±0.2 vs 3.11±0.51 mg aorta Evans Blue, P=0.0022), reduced nitric oxide availability and sustained activation and inflammation of the vascular endothelium (P<0.05). Within the atherosclerotic plaques, collagen deposition is reduced (P=0.0023) and lipid content is increased (P=0.0495), indicating enhanced plaque instability and faster disease progression. Plaque macrophages are significantly elevated and correlate with increased iron-induced CCL2 levels (12 months: 155.5±23.27 vs 305±38.39 pmol CCL2/ml serum, P=0.01), potentially contributing to increased lesion vulnerability (12 months: plaque vulnerability index P=0.0276). Ecocardiography in ApoE-/-FPNwt/C326S mice reveals an increased left ventricle mass and increased left ventricle area and volume in dyastole, plausibly as an attempt to compensate for increased arterial stiffness.Our mouse model further shows increased fibrinogen and pro-thrombin levels (P<0.05), suggesting a pro-thrombotic role for high systemic iron levels. Prolonged administration of a low-iron diet rescues the severe atherosclerotic phenotype, proving that iron is detrimental for this disease. Experiments are ongoing to test the effect of iron chelation therapy.
Conclusion
Our data suggest that high circulating iron levels strongly enhance the severity and promote the progression of atherosclerosis, indicating that systemic iron overload is a risk factor for atherosclerosis and predisposes to cardiovascular disease. Our findings have potential implications for those pathological conditions with elevated systemic iron levels, ranging from patients with hemochromatosis to anemic patients dependent on chronic blood transfusions, as well as for individuals subjected to intravenous iron administration (e.g. patients undergoing hemodialysis).
Session topic: Red blood cells and iron
Keyword(s): Atherosclerosis, Hemochromatosis, Iron chelation, Iron overload
Type: Oral Presentation
Presentation during EHA21: On Friday, June 10, 2016 from 11:45 - 12:00
Location: Hall C15
Background
Iron accumulates in atherosclerotic lesions but its role in atherogenesis is still debated. In the “iron hypothesis” (1981), Sullivan proposed that iron is detrimental for the cardiovascular system, promoting atherosclerosis progression. So far, epidemiological data and studies in animal models have provided conflicting evidence regarding a role of excess iron in atherogenesis and cardiovascular disease.
Aims
In this study we aimed to investigate the role of iron overload in the development of atherosclerosis.
Methods
To this purpose, a mouse model of type IV Hereditary Hemochromatosis, in which the hepcidin/ferroportin regulatory circuitry is disrupted due to a point mutation in the iron exporter ferroportin (FPNC326S; Altamura et al., Cell Metabolism 2014), was interbred with ApoE-null mice(ApoE-/-), that show increased susceptibility to atherosclerosis. Plaque formation was analyzed in ApoE-/-FPNwt/C326S mice at 6 and 12 months of age.
Results
ApoE-/-FPNwt/C326S mice show high serum iron and cholesterol levels, as expected. Importantly, these mice show strongly increased lesion size and number at both 6 and 12 months of age compared to age-matched Apo-/- mice (6 months: 1.44±0.23 vs 5±0.53 % aortic lesion area, P=0.0001; 12 months: 10.24±1.21 vs 20.44±2.69 % aortic lesion area, P=0.0065). The atherosclerotic phenotype positively correlates with higher levels of circulating iron (12 months: 122.2±6.57 vs 337±19.22 mg iron/dl serum, P<0.0001) and oxidized LDLs (12 months: 2151±136.8 vs 3243±193.9 nmol oxLDL/ml serum, P=0.0002).Iron is deposited in the artery media layer, which correlates with vascular smooth muscle cell senescence, calcifications, vascular oxidative stress (12 months: 2.45±0.2 vs 4.33±0.32 nmol MDA/mg aorta protein, P=0.047) and DNA damage. We observe increased vascular permeability (6 months: 0.95±0.2 vs 3.11±0.51 mg aorta Evans Blue, P=0.0022), reduced nitric oxide availability and sustained activation and inflammation of the vascular endothelium (P<0.05). Within the atherosclerotic plaques, collagen deposition is reduced (P=0.0023) and lipid content is increased (P=0.0495), indicating enhanced plaque instability and faster disease progression. Plaque macrophages are significantly elevated and correlate with increased iron-induced CCL2 levels (12 months: 155.5±23.27 vs 305±38.39 pmol CCL2/ml serum, P=0.01), potentially contributing to increased lesion vulnerability (12 months: plaque vulnerability index P=0.0276). Ecocardiography in ApoE-/-FPNwt/C326S mice reveals an increased left ventricle mass and increased left ventricle area and volume in dyastole, plausibly as an attempt to compensate for increased arterial stiffness.Our mouse model further shows increased fibrinogen and pro-thrombin levels (P<0.05), suggesting a pro-thrombotic role for high systemic iron levels. Prolonged administration of a low-iron diet rescues the severe atherosclerotic phenotype, proving that iron is detrimental for this disease. Experiments are ongoing to test the effect of iron chelation therapy.
Conclusion
Our data suggest that high circulating iron levels strongly enhance the severity and promote the progression of atherosclerosis, indicating that systemic iron overload is a risk factor for atherosclerosis and predisposes to cardiovascular disease. Our findings have potential implications for those pathological conditions with elevated systemic iron levels, ranging from patients with hemochromatosis to anemic patients dependent on chronic blood transfusions, as well as for individuals subjected to intravenous iron administration (e.g. patients undergoing hemodialysis).

Session topic: Red blood cells and iron
Keyword(s): Atherosclerosis, Hemochromatosis, Iron chelation, Iron overload
Abstract: S134
Type: Oral Presentation
Presentation during EHA21: On Friday, June 10, 2016 from 11:45 - 12:00
Location: Hall C15
Background
Iron accumulates in atherosclerotic lesions but its role in atherogenesis is still debated. In the “iron hypothesis” (1981), Sullivan proposed that iron is detrimental for the cardiovascular system, promoting atherosclerosis progression. So far, epidemiological data and studies in animal models have provided conflicting evidence regarding a role of excess iron in atherogenesis and cardiovascular disease.
Aims
In this study we aimed to investigate the role of iron overload in the development of atherosclerosis.
Methods
To this purpose, a mouse model of type IV Hereditary Hemochromatosis, in which the hepcidin/ferroportin regulatory circuitry is disrupted due to a point mutation in the iron exporter ferroportin (FPNC326S; Altamura et al., Cell Metabolism 2014), was interbred with ApoE-null mice(ApoE-/-), that show increased susceptibility to atherosclerosis. Plaque formation was analyzed in ApoE-/-FPNwt/C326S mice at 6 and 12 months of age.
Results
ApoE-/-FPNwt/C326S mice show high serum iron and cholesterol levels, as expected. Importantly, these mice show strongly increased lesion size and number at both 6 and 12 months of age compared to age-matched Apo-/- mice (6 months: 1.44±0.23 vs 5±0.53 % aortic lesion area, P=0.0001; 12 months: 10.24±1.21 vs 20.44±2.69 % aortic lesion area, P=0.0065). The atherosclerotic phenotype positively correlates with higher levels of circulating iron (12 months: 122.2±6.57 vs 337±19.22 mg iron/dl serum, P<0.0001) and oxidized LDLs (12 months: 2151±136.8 vs 3243±193.9 nmol oxLDL/ml serum, P=0.0002).Iron is deposited in the artery media layer, which correlates with vascular smooth muscle cell senescence, calcifications, vascular oxidative stress (12 months: 2.45±0.2 vs 4.33±0.32 nmol MDA/mg aorta protein, P=0.047) and DNA damage. We observe increased vascular permeability (6 months: 0.95±0.2 vs 3.11±0.51 mg aorta Evans Blue, P=0.0022), reduced nitric oxide availability and sustained activation and inflammation of the vascular endothelium (P<0.05). Within the atherosclerotic plaques, collagen deposition is reduced (P=0.0023) and lipid content is increased (P=0.0495), indicating enhanced plaque instability and faster disease progression. Plaque macrophages are significantly elevated and correlate with increased iron-induced CCL2 levels (12 months: 155.5±23.27 vs 305±38.39 pmol CCL2/ml serum, P=0.01), potentially contributing to increased lesion vulnerability (12 months: plaque vulnerability index P=0.0276). Ecocardiography in ApoE-/-FPNwt/C326S mice reveals an increased left ventricle mass and increased left ventricle area and volume in dyastole, plausibly as an attempt to compensate for increased arterial stiffness.Our mouse model further shows increased fibrinogen and pro-thrombin levels (P<0.05), suggesting a pro-thrombotic role for high systemic iron levels. Prolonged administration of a low-iron diet rescues the severe atherosclerotic phenotype, proving that iron is detrimental for this disease. Experiments are ongoing to test the effect of iron chelation therapy.
Conclusion
Our data suggest that high circulating iron levels strongly enhance the severity and promote the progression of atherosclerosis, indicating that systemic iron overload is a risk factor for atherosclerosis and predisposes to cardiovascular disease. Our findings have potential implications for those pathological conditions with elevated systemic iron levels, ranging from patients with hemochromatosis to anemic patients dependent on chronic blood transfusions, as well as for individuals subjected to intravenous iron administration (e.g. patients undergoing hemodialysis).

Session topic: Red blood cells and iron
Keyword(s): Atherosclerosis, Hemochromatosis, Iron chelation, Iron overload
Type: Oral Presentation
Presentation during EHA21: On Friday, June 10, 2016 from 11:45 - 12:00
Location: Hall C15
Background
Iron accumulates in atherosclerotic lesions but its role in atherogenesis is still debated. In the “iron hypothesis” (1981), Sullivan proposed that iron is detrimental for the cardiovascular system, promoting atherosclerosis progression. So far, epidemiological data and studies in animal models have provided conflicting evidence regarding a role of excess iron in atherogenesis and cardiovascular disease.
Aims
In this study we aimed to investigate the role of iron overload in the development of atherosclerosis.
Methods
To this purpose, a mouse model of type IV Hereditary Hemochromatosis, in which the hepcidin/ferroportin regulatory circuitry is disrupted due to a point mutation in the iron exporter ferroportin (FPNC326S; Altamura et al., Cell Metabolism 2014), was interbred with ApoE-null mice(ApoE-/-), that show increased susceptibility to atherosclerosis. Plaque formation was analyzed in ApoE-/-FPNwt/C326S mice at 6 and 12 months of age.
Results
ApoE-/-FPNwt/C326S mice show high serum iron and cholesterol levels, as expected. Importantly, these mice show strongly increased lesion size and number at both 6 and 12 months of age compared to age-matched Apo-/- mice (6 months: 1.44±0.23 vs 5±0.53 % aortic lesion area, P=0.0001; 12 months: 10.24±1.21 vs 20.44±2.69 % aortic lesion area, P=0.0065). The atherosclerotic phenotype positively correlates with higher levels of circulating iron (12 months: 122.2±6.57 vs 337±19.22 mg iron/dl serum, P<0.0001) and oxidized LDLs (12 months: 2151±136.8 vs 3243±193.9 nmol oxLDL/ml serum, P=0.0002).Iron is deposited in the artery media layer, which correlates with vascular smooth muscle cell senescence, calcifications, vascular oxidative stress (12 months: 2.45±0.2 vs 4.33±0.32 nmol MDA/mg aorta protein, P=0.047) and DNA damage. We observe increased vascular permeability (6 months: 0.95±0.2 vs 3.11±0.51 mg aorta Evans Blue, P=0.0022), reduced nitric oxide availability and sustained activation and inflammation of the vascular endothelium (P<0.05). Within the atherosclerotic plaques, collagen deposition is reduced (P=0.0023) and lipid content is increased (P=0.0495), indicating enhanced plaque instability and faster disease progression. Plaque macrophages are significantly elevated and correlate with increased iron-induced CCL2 levels (12 months: 155.5±23.27 vs 305±38.39 pmol CCL2/ml serum, P=0.01), potentially contributing to increased lesion vulnerability (12 months: plaque vulnerability index P=0.0276). Ecocardiography in ApoE-/-FPNwt/C326S mice reveals an increased left ventricle mass and increased left ventricle area and volume in dyastole, plausibly as an attempt to compensate for increased arterial stiffness.Our mouse model further shows increased fibrinogen and pro-thrombin levels (P<0.05), suggesting a pro-thrombotic role for high systemic iron levels. Prolonged administration of a low-iron diet rescues the severe atherosclerotic phenotype, proving that iron is detrimental for this disease. Experiments are ongoing to test the effect of iron chelation therapy.
Conclusion
Our data suggest that high circulating iron levels strongly enhance the severity and promote the progression of atherosclerosis, indicating that systemic iron overload is a risk factor for atherosclerosis and predisposes to cardiovascular disease. Our findings have potential implications for those pathological conditions with elevated systemic iron levels, ranging from patients with hemochromatosis to anemic patients dependent on chronic blood transfusions, as well as for individuals subjected to intravenous iron administration (e.g. patients undergoing hemodialysis).

Session topic: Red blood cells and iron
Keyword(s): Atherosclerosis, Hemochromatosis, Iron chelation, Iron overload
{{ help_message }}
{{filter}}


