THROMBOPOIETIN RECEPTOR AGONIST ELTROMBOPAG FOR ADVANCED MDS OR AML AND SEVERE THROMBOCYTOPENIA: 12-WEEK, RANDOMIZED, PLACEBO-CONTROLLED, PHASE 2 ASPIRE STUDY
(Abstract release date: 05/19/16)
EHA Library. Mittelman M. 06/10/16; 135163; S130

Dr. Moshe Mittelman
Contributions
Contributions
Abstract
Abstract: S130
Type: Oral Presentation
Presentation during EHA21: On Friday, June 10, 2016 from 12:00 - 12:15
Location: Hall C14
Background
Thrombocytopenia is a serious life-threatening complication in patients with advanced myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML). Eltrombopag (EPAG), an oral thrombopoietin receptor agonist, is approved for treatment of chronic immune thrombocytopenia, hepatitis C virus-related thrombocytopenia, and severe aplastic anemia. Preclinical studies showed that EPAG has potential antileukemic effects (Roth M et al. Blood. 2012;120:386–94). A randomized, placebo-controlled, Phase 1/2 study in advanced MDS/AML demonstrated an acceptable safety profile at EPAG doses up to 300 mg daily, with no disease progression, and a trend toward improved platelet pharmacodynamics (Platzbecker U et al. Lancet Haematol. 2015;2:e417–26).
Aims
To determine the effect of EPAG on reducing the number of clinically relevant thrombocytopenic events (CRTE) in patients with MDS or AML who have Grade 4 thrombocytopenia (platelets <25 Gi/L).
Methods
After 8 weeks of open-label, dose-defining EPAG treatment (Part 1 [Mittelman M et al. Blood. 2012;120(21): Abs 3822]), adult patients with advanced MDS or AML were randomized 2:1 in a double-blind fashion (Part 2) to 12 weeks of supportive care plus once daily EPAG (dose range 50–300 mg over the course of treatment) or placebo. Patients were stratified by baseline platelet count (<10 Gi/L vs ≥10 Gi/L) and disease severity (International Prognostic Scoring System intermediate-2/high-risk MDS versus AML). Eligibility included 10–50% baseline bone marrow blasts and a baseline platelet count of <25 Gi/L. The primary endpoint was reduction in CRTEs (a composite of platelet counts <10 Gi/L, platelet transfusions, and Grade ≥3 World Health Organization [WHO] bleeding scale events) during Weeks 5–12. Secondary endpoints included safety, platelet transfusion independence, maximum WHO bleeding, hematologic improvement, and MDS progression (increased blast percentage or leukemic transformation).
Results
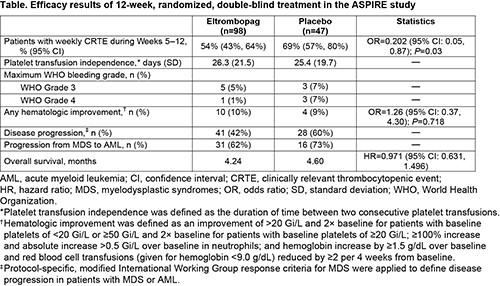
At baseline, age ranged from 29–85 years (mean 72.3; n=98) in the EPAG group vs 44–87 (mean 70.6; n=47) in the placebo group. More patients had abnormal (53% vs 34%) or poor (34% vs 17%) karyotypes in the EPAG group. Fewer EPAG patients had baseline platelets <10 Gi/L (34% vs 45%) than placebo patients. Other baseline characteristics were similar. Efficacy results are described in the Table. EPAG-treated patients showed significantly lower CRTE than placebo (54% vs 69%; odds ratio=0.202, P=0.03). Proportionately fewer patients on EPAG than placebo experienced independent reviewer-assessed disease progression (42% vs 60%). The most frequent adverse events in this study were petechiae (42% vs 23%), epistaxis (28% vs 23%), pyrexia (24% vs 28%), diarrhea (21% vs 17%), and fatigue (25% vs 9%) on EPAG versus placebo, respectively. More EPAG (31.6%) than placebo (14.9%) patients discontinued due to AEs. During Part 2, 35% of EPAG and 28% of placebo patients died (P=0.287). The primary cause of death for both groups was the disease under study (EPAG 27%; placebo 23%).
Conclusion
Treatment of patients with advanced MDS or AML with the thrombopoietin receptor agonist EPAG versus placebo for 12 weeks resulted in fewer CRTEs and did not result in an increase of disease progression. Rates of WHO Grade 3/4 bleeding were lower with EPAG. EPAG did not demonstrate overall hematologic improvement in this study. This study (NCT01440374) was sponsored by GlaxoSmithKline; however, as of March 2, 2015, eltrombopag became an asset of Novartis AG.
Session topic: Myelodysplastic syndromes - Clinical
Keyword(s): Bleeding, MDS/AML, Platelet count, Thrombopoietin (TPO)
Type: Oral Presentation
Presentation during EHA21: On Friday, June 10, 2016 from 12:00 - 12:15
Location: Hall C14
Background
Thrombocytopenia is a serious life-threatening complication in patients with advanced myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML). Eltrombopag (EPAG), an oral thrombopoietin receptor agonist, is approved for treatment of chronic immune thrombocytopenia, hepatitis C virus-related thrombocytopenia, and severe aplastic anemia. Preclinical studies showed that EPAG has potential antileukemic effects (Roth M et al. Blood. 2012;120:386–94). A randomized, placebo-controlled, Phase 1/2 study in advanced MDS/AML demonstrated an acceptable safety profile at EPAG doses up to 300 mg daily, with no disease progression, and a trend toward improved platelet pharmacodynamics (Platzbecker U et al. Lancet Haematol. 2015;2:e417–26).
Aims
To determine the effect of EPAG on reducing the number of clinically relevant thrombocytopenic events (CRTE) in patients with MDS or AML who have Grade 4 thrombocytopenia (platelets <25 Gi/L).
Methods
After 8 weeks of open-label, dose-defining EPAG treatment (Part 1 [Mittelman M et al. Blood. 2012;120(21): Abs 3822]), adult patients with advanced MDS or AML were randomized 2:1 in a double-blind fashion (Part 2) to 12 weeks of supportive care plus once daily EPAG (dose range 50–300 mg over the course of treatment) or placebo. Patients were stratified by baseline platelet count (<10 Gi/L vs ≥10 Gi/L) and disease severity (International Prognostic Scoring System intermediate-2/high-risk MDS versus AML). Eligibility included 10–50% baseline bone marrow blasts and a baseline platelet count of <25 Gi/L. The primary endpoint was reduction in CRTEs (a composite of platelet counts <10 Gi/L, platelet transfusions, and Grade ≥3 World Health Organization [WHO] bleeding scale events) during Weeks 5–12. Secondary endpoints included safety, platelet transfusion independence, maximum WHO bleeding, hematologic improvement, and MDS progression (increased blast percentage or leukemic transformation).
Results
At baseline, age ranged from 29–85 years (mean 72.3; n=98) in the EPAG group vs 44–87 (mean 70.6; n=47) in the placebo group. More patients had abnormal (53% vs 34%) or poor (34% vs 17%) karyotypes in the EPAG group. Fewer EPAG patients had baseline platelets <10 Gi/L (34% vs 45%) than placebo patients. Other baseline characteristics were similar. Efficacy results are described in the Table. EPAG-treated patients showed significantly lower CRTE than placebo (54% vs 69%; odds ratio=0.202, P=0.03). Proportionately fewer patients on EPAG than placebo experienced independent reviewer-assessed disease progression (42% vs 60%). The most frequent adverse events in this study were petechiae (42% vs 23%), epistaxis (28% vs 23%), pyrexia (24% vs 28%), diarrhea (21% vs 17%), and fatigue (25% vs 9%) on EPAG versus placebo, respectively. More EPAG (31.6%) than placebo (14.9%) patients discontinued due to AEs. During Part 2, 35% of EPAG and 28% of placebo patients died (P=0.287). The primary cause of death for both groups was the disease under study (EPAG 27%; placebo 23%).
Conclusion
Treatment of patients with advanced MDS or AML with the thrombopoietin receptor agonist EPAG versus placebo for 12 weeks resulted in fewer CRTEs and did not result in an increase of disease progression. Rates of WHO Grade 3/4 bleeding were lower with EPAG. EPAG did not demonstrate overall hematologic improvement in this study. This study (NCT01440374) was sponsored by GlaxoSmithKline; however, as of March 2, 2015, eltrombopag became an asset of Novartis AG.

Session topic: Myelodysplastic syndromes - Clinical
Keyword(s): Bleeding, MDS/AML, Platelet count, Thrombopoietin (TPO)
Abstract: S130
Type: Oral Presentation
Presentation during EHA21: On Friday, June 10, 2016 from 12:00 - 12:15
Location: Hall C14
Background
Thrombocytopenia is a serious life-threatening complication in patients with advanced myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML). Eltrombopag (EPAG), an oral thrombopoietin receptor agonist, is approved for treatment of chronic immune thrombocytopenia, hepatitis C virus-related thrombocytopenia, and severe aplastic anemia. Preclinical studies showed that EPAG has potential antileukemic effects (Roth M et al. Blood. 2012;120:386–94). A randomized, placebo-controlled, Phase 1/2 study in advanced MDS/AML demonstrated an acceptable safety profile at EPAG doses up to 300 mg daily, with no disease progression, and a trend toward improved platelet pharmacodynamics (Platzbecker U et al. Lancet Haematol. 2015;2:e417–26).
Aims
To determine the effect of EPAG on reducing the number of clinically relevant thrombocytopenic events (CRTE) in patients with MDS or AML who have Grade 4 thrombocytopenia (platelets <25 Gi/L).
Methods
After 8 weeks of open-label, dose-defining EPAG treatment (Part 1 [Mittelman M et al. Blood. 2012;120(21): Abs 3822]), adult patients with advanced MDS or AML were randomized 2:1 in a double-blind fashion (Part 2) to 12 weeks of supportive care plus once daily EPAG (dose range 50–300 mg over the course of treatment) or placebo. Patients were stratified by baseline platelet count (<10 Gi/L vs ≥10 Gi/L) and disease severity (International Prognostic Scoring System intermediate-2/high-risk MDS versus AML). Eligibility included 10–50% baseline bone marrow blasts and a baseline platelet count of <25 Gi/L. The primary endpoint was reduction in CRTEs (a composite of platelet counts <10 Gi/L, platelet transfusions, and Grade ≥3 World Health Organization [WHO] bleeding scale events) during Weeks 5–12. Secondary endpoints included safety, platelet transfusion independence, maximum WHO bleeding, hematologic improvement, and MDS progression (increased blast percentage or leukemic transformation).
Results
At baseline, age ranged from 29–85 years (mean 72.3; n=98) in the EPAG group vs 44–87 (mean 70.6; n=47) in the placebo group. More patients had abnormal (53% vs 34%) or poor (34% vs 17%) karyotypes in the EPAG group. Fewer EPAG patients had baseline platelets <10 Gi/L (34% vs 45%) than placebo patients. Other baseline characteristics were similar. Efficacy results are described in the Table. EPAG-treated patients showed significantly lower CRTE than placebo (54% vs 69%; odds ratio=0.202, P=0.03). Proportionately fewer patients on EPAG than placebo experienced independent reviewer-assessed disease progression (42% vs 60%). The most frequent adverse events in this study were petechiae (42% vs 23%), epistaxis (28% vs 23%), pyrexia (24% vs 28%), diarrhea (21% vs 17%), and fatigue (25% vs 9%) on EPAG versus placebo, respectively. More EPAG (31.6%) than placebo (14.9%) patients discontinued due to AEs. During Part 2, 35% of EPAG and 28% of placebo patients died (P=0.287). The primary cause of death for both groups was the disease under study (EPAG 27%; placebo 23%).
Conclusion
Treatment of patients with advanced MDS or AML with the thrombopoietin receptor agonist EPAG versus placebo for 12 weeks resulted in fewer CRTEs and did not result in an increase of disease progression. Rates of WHO Grade 3/4 bleeding were lower with EPAG. EPAG did not demonstrate overall hematologic improvement in this study. This study (NCT01440374) was sponsored by GlaxoSmithKline; however, as of March 2, 2015, eltrombopag became an asset of Novartis AG.

Session topic: Myelodysplastic syndromes - Clinical
Keyword(s): Bleeding, MDS/AML, Platelet count, Thrombopoietin (TPO)
Type: Oral Presentation
Presentation during EHA21: On Friday, June 10, 2016 from 12:00 - 12:15
Location: Hall C14
Background
Thrombocytopenia is a serious life-threatening complication in patients with advanced myelodysplastic syndromes (MDS) and acute myeloid leukemia (AML). Eltrombopag (EPAG), an oral thrombopoietin receptor agonist, is approved for treatment of chronic immune thrombocytopenia, hepatitis C virus-related thrombocytopenia, and severe aplastic anemia. Preclinical studies showed that EPAG has potential antileukemic effects (Roth M et al. Blood. 2012;120:386–94). A randomized, placebo-controlled, Phase 1/2 study in advanced MDS/AML demonstrated an acceptable safety profile at EPAG doses up to 300 mg daily, with no disease progression, and a trend toward improved platelet pharmacodynamics (Platzbecker U et al. Lancet Haematol. 2015;2:e417–26).
Aims
To determine the effect of EPAG on reducing the number of clinically relevant thrombocytopenic events (CRTE) in patients with MDS or AML who have Grade 4 thrombocytopenia (platelets <25 Gi/L).
Methods
After 8 weeks of open-label, dose-defining EPAG treatment (Part 1 [Mittelman M et al. Blood. 2012;120(21): Abs 3822]), adult patients with advanced MDS or AML were randomized 2:1 in a double-blind fashion (Part 2) to 12 weeks of supportive care plus once daily EPAG (dose range 50–300 mg over the course of treatment) or placebo. Patients were stratified by baseline platelet count (<10 Gi/L vs ≥10 Gi/L) and disease severity (International Prognostic Scoring System intermediate-2/high-risk MDS versus AML). Eligibility included 10–50% baseline bone marrow blasts and a baseline platelet count of <25 Gi/L. The primary endpoint was reduction in CRTEs (a composite of platelet counts <10 Gi/L, platelet transfusions, and Grade ≥3 World Health Organization [WHO] bleeding scale events) during Weeks 5–12. Secondary endpoints included safety, platelet transfusion independence, maximum WHO bleeding, hematologic improvement, and MDS progression (increased blast percentage or leukemic transformation).
Results
At baseline, age ranged from 29–85 years (mean 72.3; n=98) in the EPAG group vs 44–87 (mean 70.6; n=47) in the placebo group. More patients had abnormal (53% vs 34%) or poor (34% vs 17%) karyotypes in the EPAG group. Fewer EPAG patients had baseline platelets <10 Gi/L (34% vs 45%) than placebo patients. Other baseline characteristics were similar. Efficacy results are described in the Table. EPAG-treated patients showed significantly lower CRTE than placebo (54% vs 69%; odds ratio=0.202, P=0.03). Proportionately fewer patients on EPAG than placebo experienced independent reviewer-assessed disease progression (42% vs 60%). The most frequent adverse events in this study were petechiae (42% vs 23%), epistaxis (28% vs 23%), pyrexia (24% vs 28%), diarrhea (21% vs 17%), and fatigue (25% vs 9%) on EPAG versus placebo, respectively. More EPAG (31.6%) than placebo (14.9%) patients discontinued due to AEs. During Part 2, 35% of EPAG and 28% of placebo patients died (P=0.287). The primary cause of death for both groups was the disease under study (EPAG 27%; placebo 23%).
Conclusion
Treatment of patients with advanced MDS or AML with the thrombopoietin receptor agonist EPAG versus placebo for 12 weeks resulted in fewer CRTEs and did not result in an increase of disease progression. Rates of WHO Grade 3/4 bleeding were lower with EPAG. EPAG did not demonstrate overall hematologic improvement in this study. This study (NCT01440374) was sponsored by GlaxoSmithKline; however, as of March 2, 2015, eltrombopag became an asset of Novartis AG.

Session topic: Myelodysplastic syndromes - Clinical
Keyword(s): Bleeding, MDS/AML, Platelet count, Thrombopoietin (TPO)
{{ help_message }}
{{filter}}


