HNRNP K: AN ONCOGENE AND TUMOR SUPPRESSOR, TWO DISCRETE PATHS TO AML THROUGH ON GENE
(Abstract release date: 05/19/16)
EHA Library. Gallardo M. 06/10/16; 135153; S120

Dr. Miguel Gallardo
Contributions
Contributions
Abstract
Abstract: S120
Type: Oral Presentation
Presentation during EHA21: On Friday, June 10, 2016 from 11:45 - 12:00
Location: Hall A3
Background
We have recently demonstrated that HNRNPK is a critical haploinsufficient tumor suppressor residing at the 9q21.32 locus that is deleted in a subset of patients with AML (Gallardo et al, Cancer Cell, 2016).In this study, we generated a haploinsufficient Hnrnpk (Hnrnpk+/-) mouse model that exhibited reduced survival and developed significant hematologic and myeloid malignancies. However, in addition to HNRNPK haploinsufficiency, we found that a large subset of AML patients without a 9q21.32 deletion, actually overexpress HNRNP K, resulting in poor prognoses. These data suggest that hnRNP K may have both tumor suppressive or oncogenic functions, depending on its expression levels.
Aims
This work will investigate the role of hnRNP K in AML, and to understand how HNRNP K behaves as an oncogene or as a tumor suppressor.
Methods
We analyzed copy number variations and aberrant expression of HNRNPK by FISH, qRT-PCR, and RPPA analyses in AML patients with and without 9q21.32 deletions. To directly examine the contribution of aberrant hnRNP K expression to malignant phenotypes, we generated two distinct cohorts of genetically engineered mice. One cohort was haploinsufficient for Hnrnpk, while the other specifically overexpressed hnRNP K in hematopoietic progenitors (HnrnpkTg). Differences in survival, genomic stability, proliferation and differentiation potential, tumor formation, and molecular analyses were performed on hematological tissues using qRT-PCR, immunohistochemistry, colony formation assays, western blot analyses, and transplantation assays. Molecules of interest were further analyzed for interaction with hnRNP K via ChIP and RIP assays.
Results
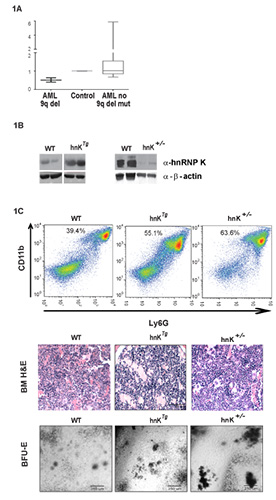
Initial evaluation of HNRNPK expression levels in AML patients revealed that patients with the 9q21.32 deletion exhibited reduced hnRNP K levels, while many patients without this deletion had significant HNRNPK overexpression (Fig. 1A). This result was echoed in FISH analyses, as patients with the 9q21.32 deletion showed HNRNPK loss, while nearly 37% of AML (n = 43) patients without the 9q deletion showed gene amplificationsIn order to investigate the role of HNRNP K in AML, we generated both hnRNP K+/- and hnRNP Ktg mice. While these two mouse models have significant differences in hnRNP K expression compared to wild type (reduced versus overexpression), they surprisingly, had extremely similar phenotypes (Fig. 1B). Mice from each cohort suffered reduced survival, development of myeloid malignancies with high penetrance, genomic instability, enhancement in proliferation and differentiation potential in HSPCs, and the ability to generate myeloid hyperplasias following transplantation (Fig. 1C).Even though these opposite hnRNP K expression patterns result in extremely similar phenotypes, the molecular consequence of aberrant hnRNP K expression is facilitated through discrete molecular mechanisms. While hnRNP K haploinsufficiency directly decreased expression of anti-proliferation and differentiation genes likes p21, C/EBPa, and C/EBPβ, its overexpression resulted in the direct activation of pro-growth genes like c-Myc. Given that hnRNP K expression is known to be tightly in all eukarotyic organisms, these data suggest that any alteration in hnRNP K expression may result in drastic cellular consequences.
Conclusion
These data provide evidence that hnRNP K has the unique capacity to behave as an oncogene when overexpressed, or as a tumor suppressor when is its expression is reduced. In either case, these imbalances in hnRNP K expression are capable of directly contributing to the pathogenesis of AML.
Session topic: AML Biology - Novel mechanisms of leukemogenesis
Keyword(s): Acute myeloid leukemia, Oncogene, Tumor suppressor
Type: Oral Presentation
Presentation during EHA21: On Friday, June 10, 2016 from 11:45 - 12:00
Location: Hall A3
Background
We have recently demonstrated that HNRNPK is a critical haploinsufficient tumor suppressor residing at the 9q21.32 locus that is deleted in a subset of patients with AML (Gallardo et al, Cancer Cell, 2016).In this study, we generated a haploinsufficient Hnrnpk (Hnrnpk+/-) mouse model that exhibited reduced survival and developed significant hematologic and myeloid malignancies. However, in addition to HNRNPK haploinsufficiency, we found that a large subset of AML patients without a 9q21.32 deletion, actually overexpress HNRNP K, resulting in poor prognoses. These data suggest that hnRNP K may have both tumor suppressive or oncogenic functions, depending on its expression levels.
Aims
This work will investigate the role of hnRNP K in AML, and to understand how HNRNP K behaves as an oncogene or as a tumor suppressor.
Methods
We analyzed copy number variations and aberrant expression of HNRNPK by FISH, qRT-PCR, and RPPA analyses in AML patients with and without 9q21.32 deletions. To directly examine the contribution of aberrant hnRNP K expression to malignant phenotypes, we generated two distinct cohorts of genetically engineered mice. One cohort was haploinsufficient for Hnrnpk, while the other specifically overexpressed hnRNP K in hematopoietic progenitors (HnrnpkTg). Differences in survival, genomic stability, proliferation and differentiation potential, tumor formation, and molecular analyses were performed on hematological tissues using qRT-PCR, immunohistochemistry, colony formation assays, western blot analyses, and transplantation assays. Molecules of interest were further analyzed for interaction with hnRNP K via ChIP and RIP assays.
Results
Initial evaluation of HNRNPK expression levels in AML patients revealed that patients with the 9q21.32 deletion exhibited reduced hnRNP K levels, while many patients without this deletion had significant HNRNPK overexpression (Fig. 1A). This result was echoed in FISH analyses, as patients with the 9q21.32 deletion showed HNRNPK loss, while nearly 37% of AML (n = 43) patients without the 9q deletion showed gene amplificationsIn order to investigate the role of HNRNP K in AML, we generated both hnRNP K+/- and hnRNP Ktg mice. While these two mouse models have significant differences in hnRNP K expression compared to wild type (reduced versus overexpression), they surprisingly, had extremely similar phenotypes (Fig. 1B). Mice from each cohort suffered reduced survival, development of myeloid malignancies with high penetrance, genomic instability, enhancement in proliferation and differentiation potential in HSPCs, and the ability to generate myeloid hyperplasias following transplantation (Fig. 1C).Even though these opposite hnRNP K expression patterns result in extremely similar phenotypes, the molecular consequence of aberrant hnRNP K expression is facilitated through discrete molecular mechanisms. While hnRNP K haploinsufficiency directly decreased expression of anti-proliferation and differentiation genes likes p21, C/EBPa, and C/EBPβ, its overexpression resulted in the direct activation of pro-growth genes like c-Myc. Given that hnRNP K expression is known to be tightly in all eukarotyic organisms, these data suggest that any alteration in hnRNP K expression may result in drastic cellular consequences.
Conclusion
These data provide evidence that hnRNP K has the unique capacity to behave as an oncogene when overexpressed, or as a tumor suppressor when is its expression is reduced. In either case, these imbalances in hnRNP K expression are capable of directly contributing to the pathogenesis of AML.

Session topic: AML Biology - Novel mechanisms of leukemogenesis
Keyword(s): Acute myeloid leukemia, Oncogene, Tumor suppressor
Abstract: S120
Type: Oral Presentation
Presentation during EHA21: On Friday, June 10, 2016 from 11:45 - 12:00
Location: Hall A3
Background
We have recently demonstrated that HNRNPK is a critical haploinsufficient tumor suppressor residing at the 9q21.32 locus that is deleted in a subset of patients with AML (Gallardo et al, Cancer Cell, 2016).In this study, we generated a haploinsufficient Hnrnpk (Hnrnpk+/-) mouse model that exhibited reduced survival and developed significant hematologic and myeloid malignancies. However, in addition to HNRNPK haploinsufficiency, we found that a large subset of AML patients without a 9q21.32 deletion, actually overexpress HNRNP K, resulting in poor prognoses. These data suggest that hnRNP K may have both tumor suppressive or oncogenic functions, depending on its expression levels.
Aims
This work will investigate the role of hnRNP K in AML, and to understand how HNRNP K behaves as an oncogene or as a tumor suppressor.
Methods
We analyzed copy number variations and aberrant expression of HNRNPK by FISH, qRT-PCR, and RPPA analyses in AML patients with and without 9q21.32 deletions. To directly examine the contribution of aberrant hnRNP K expression to malignant phenotypes, we generated two distinct cohorts of genetically engineered mice. One cohort was haploinsufficient for Hnrnpk, while the other specifically overexpressed hnRNP K in hematopoietic progenitors (HnrnpkTg). Differences in survival, genomic stability, proliferation and differentiation potential, tumor formation, and molecular analyses were performed on hematological tissues using qRT-PCR, immunohistochemistry, colony formation assays, western blot analyses, and transplantation assays. Molecules of interest were further analyzed for interaction with hnRNP K via ChIP and RIP assays.
Results
Initial evaluation of HNRNPK expression levels in AML patients revealed that patients with the 9q21.32 deletion exhibited reduced hnRNP K levels, while many patients without this deletion had significant HNRNPK overexpression (Fig. 1A). This result was echoed in FISH analyses, as patients with the 9q21.32 deletion showed HNRNPK loss, while nearly 37% of AML (n = 43) patients without the 9q deletion showed gene amplificationsIn order to investigate the role of HNRNP K in AML, we generated both hnRNP K+/- and hnRNP Ktg mice. While these two mouse models have significant differences in hnRNP K expression compared to wild type (reduced versus overexpression), they surprisingly, had extremely similar phenotypes (Fig. 1B). Mice from each cohort suffered reduced survival, development of myeloid malignancies with high penetrance, genomic instability, enhancement in proliferation and differentiation potential in HSPCs, and the ability to generate myeloid hyperplasias following transplantation (Fig. 1C).Even though these opposite hnRNP K expression patterns result in extremely similar phenotypes, the molecular consequence of aberrant hnRNP K expression is facilitated through discrete molecular mechanisms. While hnRNP K haploinsufficiency directly decreased expression of anti-proliferation and differentiation genes likes p21, C/EBPa, and C/EBPβ, its overexpression resulted in the direct activation of pro-growth genes like c-Myc. Given that hnRNP K expression is known to be tightly in all eukarotyic organisms, these data suggest that any alteration in hnRNP K expression may result in drastic cellular consequences.
Conclusion
These data provide evidence that hnRNP K has the unique capacity to behave as an oncogene when overexpressed, or as a tumor suppressor when is its expression is reduced. In either case, these imbalances in hnRNP K expression are capable of directly contributing to the pathogenesis of AML.

Session topic: AML Biology - Novel mechanisms of leukemogenesis
Keyword(s): Acute myeloid leukemia, Oncogene, Tumor suppressor
Type: Oral Presentation
Presentation during EHA21: On Friday, June 10, 2016 from 11:45 - 12:00
Location: Hall A3
Background
We have recently demonstrated that HNRNPK is a critical haploinsufficient tumor suppressor residing at the 9q21.32 locus that is deleted in a subset of patients with AML (Gallardo et al, Cancer Cell, 2016).In this study, we generated a haploinsufficient Hnrnpk (Hnrnpk+/-) mouse model that exhibited reduced survival and developed significant hematologic and myeloid malignancies. However, in addition to HNRNPK haploinsufficiency, we found that a large subset of AML patients without a 9q21.32 deletion, actually overexpress HNRNP K, resulting in poor prognoses. These data suggest that hnRNP K may have both tumor suppressive or oncogenic functions, depending on its expression levels.
Aims
This work will investigate the role of hnRNP K in AML, and to understand how HNRNP K behaves as an oncogene or as a tumor suppressor.
Methods
We analyzed copy number variations and aberrant expression of HNRNPK by FISH, qRT-PCR, and RPPA analyses in AML patients with and without 9q21.32 deletions. To directly examine the contribution of aberrant hnRNP K expression to malignant phenotypes, we generated two distinct cohorts of genetically engineered mice. One cohort was haploinsufficient for Hnrnpk, while the other specifically overexpressed hnRNP K in hematopoietic progenitors (HnrnpkTg). Differences in survival, genomic stability, proliferation and differentiation potential, tumor formation, and molecular analyses were performed on hematological tissues using qRT-PCR, immunohistochemistry, colony formation assays, western blot analyses, and transplantation assays. Molecules of interest were further analyzed for interaction with hnRNP K via ChIP and RIP assays.
Results
Initial evaluation of HNRNPK expression levels in AML patients revealed that patients with the 9q21.32 deletion exhibited reduced hnRNP K levels, while many patients without this deletion had significant HNRNPK overexpression (Fig. 1A). This result was echoed in FISH analyses, as patients with the 9q21.32 deletion showed HNRNPK loss, while nearly 37% of AML (n = 43) patients without the 9q deletion showed gene amplificationsIn order to investigate the role of HNRNP K in AML, we generated both hnRNP K+/- and hnRNP Ktg mice. While these two mouse models have significant differences in hnRNP K expression compared to wild type (reduced versus overexpression), they surprisingly, had extremely similar phenotypes (Fig. 1B). Mice from each cohort suffered reduced survival, development of myeloid malignancies with high penetrance, genomic instability, enhancement in proliferation and differentiation potential in HSPCs, and the ability to generate myeloid hyperplasias following transplantation (Fig. 1C).Even though these opposite hnRNP K expression patterns result in extremely similar phenotypes, the molecular consequence of aberrant hnRNP K expression is facilitated through discrete molecular mechanisms. While hnRNP K haploinsufficiency directly decreased expression of anti-proliferation and differentiation genes likes p21, C/EBPa, and C/EBPβ, its overexpression resulted in the direct activation of pro-growth genes like c-Myc. Given that hnRNP K expression is known to be tightly in all eukarotyic organisms, these data suggest that any alteration in hnRNP K expression may result in drastic cellular consequences.
Conclusion
These data provide evidence that hnRNP K has the unique capacity to behave as an oncogene when overexpressed, or as a tumor suppressor when is its expression is reduced. In either case, these imbalances in hnRNP K expression are capable of directly contributing to the pathogenesis of AML.

Session topic: AML Biology - Novel mechanisms of leukemogenesis
Keyword(s): Acute myeloid leukemia, Oncogene, Tumor suppressor
{{ help_message }}
{{filter}}


