RUXOLITINIB PROVES SUPERIOR TO BEST AVAILABLE THERAPY IN PATIENTS WITH POLYCYTHEMIA VERA (PV) AND A NONPALPABLE SPLEEN: RESULTS FROM THE PHASE IIIB RESPONSE-2 STUDY
(Abstract release date: 05/19/16)
EHA Library. Passamonti F. 06/10/16; 135145; S112

Prof. Francesco Passamonti
Contributions
Contributions
Abstract
Abstract: S112
Type: Oral Presentation
Presentation during EHA21: On Friday, June 10, 2016 from 12:00 - 12:15
Location: Auditorium 1
Background
PV is a myeloproliferative neoplasm characterized by erythrocytosis, burdensome symptoms, and increased risk of thrombosis. A key treatment goal is to maintain hematocrit (HCT) control. In the phase 3 RESPONSE study, the JAK1/JAK2 inhibitor ruxolitinib was superior to best available therapy (BAT) in maintaining HCT control without phlebotomy (PBT), normalizing blood cell count, reducing spleen volume, and improving symptoms in HU-resistant/intolerant PV patients (pts) with splenomegaly.
Aims
RESPONSE-2 is an open-label phase 3b study comparing RUX with BAT in HU-resistant/intolerant PV pts without palpable splenomegaly.
Methods
HU-resistant/intolerant pts without palpable splenomegaly who required PBT for HCT control were randomized 1:1 to RUX 10 mg bid or BAT. The primary endpoint was the proportion of pts who achieved HCT control at wk 28 (defined as the absence of PBT eligibility [HCT >45% and ≥3 percentage points from baseline, or HCT >48%] from wk 8 to 28, with ≤1 PBT eligibility from wk 0 to 8). The key secondary endpoint was the proportion of pts who achieved complete hematologic remission (CHR) at wk 28. Other endpoints included patient-reported outcomes and safety. The MPN-SAF TSS was used to assess 10 PV-related symptoms, each on a scale of 0 (absent) to 10 (worst imaginable). BAT pts could cross over to RUX from wk 28. Primary analysis occurred when all pts reached wk 28 or discontinued.
Results
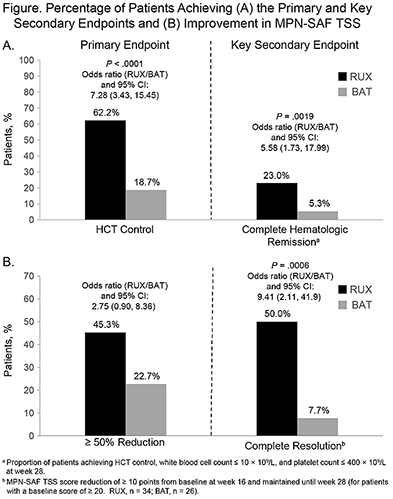
74 and 75 pts were randomized to RUX and BAT, respectively. The median time since PV diagnosis was 6.5 and 6.7 y; 28% and 24% of pts had a history of thromboembolic events; and 78% and 76% had ≥2 PBTs within 24 wk of screening, respectively. Overall, 29.5% of pts received >1 prior line of PV-directed therapy. By the cutoff date (29 Sep 2015), 2 (3%) RUX and 56 (75%) BAT pts had discontinued randomized treatment, of which 1.4% and 8.0% were due to adverse events (AEs), respectively; 51 (68%) pts crossed over to RUX. The median duration of exposure to RUX and BAT was 42 and 28 wk, respectively; median dose intensity for RUX was 20 mg/day. HCT control (primary endpoint) was achieved in 62% of RUX vs 19% of BAT pts (P<.0001); CHR (key secondary endpoint) was achieved in 23% and 5% of RUX and BAT pts, respectively (P=.0019; Fig A). At wk 28, 45% of RUX pts, vs 23% of BAT pts, had ≥50% improvement in MPN-SAF TSS; 50% vs 7.7% achieved complete symptom resolution (Fig B). RUX pts had improvements in individual symptoms; most symptoms worsened with BAT. Pruritus improved in RUX pts, but did not change in most BAT pts; 60% of RUX vs 5.3% of BAT pts had a much or very much improved condition in PGIC. Anemia or thrombocytopenia occurred in 16.2% and 2.7% of pts in the RUX arm vs 2.7% and 8.0% in the BAT arm. Corresponding grade 3/4 events were reported in 0% of RUX pts vs 1.3% and 4.0% of BAT pts, respectively. The most common nonhematologic AEs (>10% of pts) in either the RUX or BAT arm were headache (12.2%; 10.7%), constipation (10.8%; 5.3%), hypertension (10.8%; 4.0%), pruritus (10.8%; 20.0%), and weight increase (10.8%; 1.3%). All were grade 1/2, except hypertension (6.8%; 4.0%) and pruritus (0%; 2.7%). No deaths were reported in the RUX arm; 2 (2.7%) occurred in the BAT arm.
Conclusion
In this study, RUX was well tolerated and superior to BAT in controlling HCT without PBT, normalizing blood counts, and improving PV-related symptoms in pts with PV resistant to or intolerant of HU, thus extending the results from RESPONSE to pts without palpable splenomegaly.
Session topic: Myeloproliferative neoplasms - Clinical 1
Keyword(s): Polycythemia vera
Type: Oral Presentation
Presentation during EHA21: On Friday, June 10, 2016 from 12:00 - 12:15
Location: Auditorium 1
Background
PV is a myeloproliferative neoplasm characterized by erythrocytosis, burdensome symptoms, and increased risk of thrombosis. A key treatment goal is to maintain hematocrit (HCT) control. In the phase 3 RESPONSE study, the JAK1/JAK2 inhibitor ruxolitinib was superior to best available therapy (BAT) in maintaining HCT control without phlebotomy (PBT), normalizing blood cell count, reducing spleen volume, and improving symptoms in HU-resistant/intolerant PV patients (pts) with splenomegaly.
Aims
RESPONSE-2 is an open-label phase 3b study comparing RUX with BAT in HU-resistant/intolerant PV pts without palpable splenomegaly.
Methods
HU-resistant/intolerant pts without palpable splenomegaly who required PBT for HCT control were randomized 1:1 to RUX 10 mg bid or BAT. The primary endpoint was the proportion of pts who achieved HCT control at wk 28 (defined as the absence of PBT eligibility [HCT >45% and ≥3 percentage points from baseline, or HCT >48%] from wk 8 to 28, with ≤1 PBT eligibility from wk 0 to 8). The key secondary endpoint was the proportion of pts who achieved complete hematologic remission (CHR) at wk 28. Other endpoints included patient-reported outcomes and safety. The MPN-SAF TSS was used to assess 10 PV-related symptoms, each on a scale of 0 (absent) to 10 (worst imaginable). BAT pts could cross over to RUX from wk 28. Primary analysis occurred when all pts reached wk 28 or discontinued.
Results
74 and 75 pts were randomized to RUX and BAT, respectively. The median time since PV diagnosis was 6.5 and 6.7 y; 28% and 24% of pts had a history of thromboembolic events; and 78% and 76% had ≥2 PBTs within 24 wk of screening, respectively. Overall, 29.5% of pts received >1 prior line of PV-directed therapy. By the cutoff date (29 Sep 2015), 2 (3%) RUX and 56 (75%) BAT pts had discontinued randomized treatment, of which 1.4% and 8.0% were due to adverse events (AEs), respectively; 51 (68%) pts crossed over to RUX. The median duration of exposure to RUX and BAT was 42 and 28 wk, respectively; median dose intensity for RUX was 20 mg/day. HCT control (primary endpoint) was achieved in 62% of RUX vs 19% of BAT pts (P<.0001); CHR (key secondary endpoint) was achieved in 23% and 5% of RUX and BAT pts, respectively (P=.0019; Fig A). At wk 28, 45% of RUX pts, vs 23% of BAT pts, had ≥50% improvement in MPN-SAF TSS; 50% vs 7.7% achieved complete symptom resolution (Fig B). RUX pts had improvements in individual symptoms; most symptoms worsened with BAT. Pruritus improved in RUX pts, but did not change in most BAT pts; 60% of RUX vs 5.3% of BAT pts had a much or very much improved condition in PGIC. Anemia or thrombocytopenia occurred in 16.2% and 2.7% of pts in the RUX arm vs 2.7% and 8.0% in the BAT arm. Corresponding grade 3/4 events were reported in 0% of RUX pts vs 1.3% and 4.0% of BAT pts, respectively. The most common nonhematologic AEs (>10% of pts) in either the RUX or BAT arm were headache (12.2%; 10.7%), constipation (10.8%; 5.3%), hypertension (10.8%; 4.0%), pruritus (10.8%; 20.0%), and weight increase (10.8%; 1.3%). All were grade 1/2, except hypertension (6.8%; 4.0%) and pruritus (0%; 2.7%). No deaths were reported in the RUX arm; 2 (2.7%) occurred in the BAT arm.
Conclusion
In this study, RUX was well tolerated and superior to BAT in controlling HCT without PBT, normalizing blood counts, and improving PV-related symptoms in pts with PV resistant to or intolerant of HU, thus extending the results from RESPONSE to pts without palpable splenomegaly.

Session topic: Myeloproliferative neoplasms - Clinical 1
Keyword(s): Polycythemia vera
Abstract: S112
Type: Oral Presentation
Presentation during EHA21: On Friday, June 10, 2016 from 12:00 - 12:15
Location: Auditorium 1
Background
PV is a myeloproliferative neoplasm characterized by erythrocytosis, burdensome symptoms, and increased risk of thrombosis. A key treatment goal is to maintain hematocrit (HCT) control. In the phase 3 RESPONSE study, the JAK1/JAK2 inhibitor ruxolitinib was superior to best available therapy (BAT) in maintaining HCT control without phlebotomy (PBT), normalizing blood cell count, reducing spleen volume, and improving symptoms in HU-resistant/intolerant PV patients (pts) with splenomegaly.
Aims
RESPONSE-2 is an open-label phase 3b study comparing RUX with BAT in HU-resistant/intolerant PV pts without palpable splenomegaly.
Methods
HU-resistant/intolerant pts without palpable splenomegaly who required PBT for HCT control were randomized 1:1 to RUX 10 mg bid or BAT. The primary endpoint was the proportion of pts who achieved HCT control at wk 28 (defined as the absence of PBT eligibility [HCT >45% and ≥3 percentage points from baseline, or HCT >48%] from wk 8 to 28, with ≤1 PBT eligibility from wk 0 to 8). The key secondary endpoint was the proportion of pts who achieved complete hematologic remission (CHR) at wk 28. Other endpoints included patient-reported outcomes and safety. The MPN-SAF TSS was used to assess 10 PV-related symptoms, each on a scale of 0 (absent) to 10 (worst imaginable). BAT pts could cross over to RUX from wk 28. Primary analysis occurred when all pts reached wk 28 or discontinued.
Results
74 and 75 pts were randomized to RUX and BAT, respectively. The median time since PV diagnosis was 6.5 and 6.7 y; 28% and 24% of pts had a history of thromboembolic events; and 78% and 76% had ≥2 PBTs within 24 wk of screening, respectively. Overall, 29.5% of pts received >1 prior line of PV-directed therapy. By the cutoff date (29 Sep 2015), 2 (3%) RUX and 56 (75%) BAT pts had discontinued randomized treatment, of which 1.4% and 8.0% were due to adverse events (AEs), respectively; 51 (68%) pts crossed over to RUX. The median duration of exposure to RUX and BAT was 42 and 28 wk, respectively; median dose intensity for RUX was 20 mg/day. HCT control (primary endpoint) was achieved in 62% of RUX vs 19% of BAT pts (P<.0001); CHR (key secondary endpoint) was achieved in 23% and 5% of RUX and BAT pts, respectively (P=.0019; Fig A). At wk 28, 45% of RUX pts, vs 23% of BAT pts, had ≥50% improvement in MPN-SAF TSS; 50% vs 7.7% achieved complete symptom resolution (Fig B). RUX pts had improvements in individual symptoms; most symptoms worsened with BAT. Pruritus improved in RUX pts, but did not change in most BAT pts; 60% of RUX vs 5.3% of BAT pts had a much or very much improved condition in PGIC. Anemia or thrombocytopenia occurred in 16.2% and 2.7% of pts in the RUX arm vs 2.7% and 8.0% in the BAT arm. Corresponding grade 3/4 events were reported in 0% of RUX pts vs 1.3% and 4.0% of BAT pts, respectively. The most common nonhematologic AEs (>10% of pts) in either the RUX or BAT arm were headache (12.2%; 10.7%), constipation (10.8%; 5.3%), hypertension (10.8%; 4.0%), pruritus (10.8%; 20.0%), and weight increase (10.8%; 1.3%). All were grade 1/2, except hypertension (6.8%; 4.0%) and pruritus (0%; 2.7%). No deaths were reported in the RUX arm; 2 (2.7%) occurred in the BAT arm.
Conclusion
In this study, RUX was well tolerated and superior to BAT in controlling HCT without PBT, normalizing blood counts, and improving PV-related symptoms in pts with PV resistant to or intolerant of HU, thus extending the results from RESPONSE to pts without palpable splenomegaly.

Session topic: Myeloproliferative neoplasms - Clinical 1
Keyword(s): Polycythemia vera
Type: Oral Presentation
Presentation during EHA21: On Friday, June 10, 2016 from 12:00 - 12:15
Location: Auditorium 1
Background
PV is a myeloproliferative neoplasm characterized by erythrocytosis, burdensome symptoms, and increased risk of thrombosis. A key treatment goal is to maintain hematocrit (HCT) control. In the phase 3 RESPONSE study, the JAK1/JAK2 inhibitor ruxolitinib was superior to best available therapy (BAT) in maintaining HCT control without phlebotomy (PBT), normalizing blood cell count, reducing spleen volume, and improving symptoms in HU-resistant/intolerant PV patients (pts) with splenomegaly.
Aims
RESPONSE-2 is an open-label phase 3b study comparing RUX with BAT in HU-resistant/intolerant PV pts without palpable splenomegaly.
Methods
HU-resistant/intolerant pts without palpable splenomegaly who required PBT for HCT control were randomized 1:1 to RUX 10 mg bid or BAT. The primary endpoint was the proportion of pts who achieved HCT control at wk 28 (defined as the absence of PBT eligibility [HCT >45% and ≥3 percentage points from baseline, or HCT >48%] from wk 8 to 28, with ≤1 PBT eligibility from wk 0 to 8). The key secondary endpoint was the proportion of pts who achieved complete hematologic remission (CHR) at wk 28. Other endpoints included patient-reported outcomes and safety. The MPN-SAF TSS was used to assess 10 PV-related symptoms, each on a scale of 0 (absent) to 10 (worst imaginable). BAT pts could cross over to RUX from wk 28. Primary analysis occurred when all pts reached wk 28 or discontinued.
Results
74 and 75 pts were randomized to RUX and BAT, respectively. The median time since PV diagnosis was 6.5 and 6.7 y; 28% and 24% of pts had a history of thromboembolic events; and 78% and 76% had ≥2 PBTs within 24 wk of screening, respectively. Overall, 29.5% of pts received >1 prior line of PV-directed therapy. By the cutoff date (29 Sep 2015), 2 (3%) RUX and 56 (75%) BAT pts had discontinued randomized treatment, of which 1.4% and 8.0% were due to adverse events (AEs), respectively; 51 (68%) pts crossed over to RUX. The median duration of exposure to RUX and BAT was 42 and 28 wk, respectively; median dose intensity for RUX was 20 mg/day. HCT control (primary endpoint) was achieved in 62% of RUX vs 19% of BAT pts (P<.0001); CHR (key secondary endpoint) was achieved in 23% and 5% of RUX and BAT pts, respectively (P=.0019; Fig A). At wk 28, 45% of RUX pts, vs 23% of BAT pts, had ≥50% improvement in MPN-SAF TSS; 50% vs 7.7% achieved complete symptom resolution (Fig B). RUX pts had improvements in individual symptoms; most symptoms worsened with BAT. Pruritus improved in RUX pts, but did not change in most BAT pts; 60% of RUX vs 5.3% of BAT pts had a much or very much improved condition in PGIC. Anemia or thrombocytopenia occurred in 16.2% and 2.7% of pts in the RUX arm vs 2.7% and 8.0% in the BAT arm. Corresponding grade 3/4 events were reported in 0% of RUX pts vs 1.3% and 4.0% of BAT pts, respectively. The most common nonhematologic AEs (>10% of pts) in either the RUX or BAT arm were headache (12.2%; 10.7%), constipation (10.8%; 5.3%), hypertension (10.8%; 4.0%), pruritus (10.8%; 20.0%), and weight increase (10.8%; 1.3%). All were grade 1/2, except hypertension (6.8%; 4.0%) and pruritus (0%; 2.7%). No deaths were reported in the RUX arm; 2 (2.7%) occurred in the BAT arm.
Conclusion
In this study, RUX was well tolerated and superior to BAT in controlling HCT without PBT, normalizing blood counts, and improving PV-related symptoms in pts with PV resistant to or intolerant of HU, thus extending the results from RESPONSE to pts without palpable splenomegaly.

Session topic: Myeloproliferative neoplasms - Clinical 1
Keyword(s): Polycythemia vera
{{ help_message }}
{{filter}}


